Efficacy of additional ExTRa Mapping-guided substrate ablation beyond pulmonary vein isolation in persistent atrial fibrillation: The ROTATE trial
- PMID: 40548679
- PMCID: PMC12337631
- DOI: 10.1111/jce.16772
Efficacy of additional ExTRa Mapping-guided substrate ablation beyond pulmonary vein isolation in persistent atrial fibrillation: The ROTATE trial
Abstract
Introduction: There are currently no established effective additional substrate ablation strategies beyond pulmonary vein isolation (PVI) for persistent atrial fibrillation (AF).
Objective: This randomized clinical trial evaluated the efficacy of a novel substrate ablation technique using the ExTRa Mapping system, which visualizes rotational activation during AF rhythm.
Methods: This study included 80 patients undergoing initial catheter ablation for persistent AF. Eighty patients whose AF persisted after PVI and ExTRa Mapping were randomly assigned in a 1:1 ratio to either PVI alone or PVI plus ExTRa Mapping-guided substrate ablation targeting areas with high non-passively activated ratio(%NP)( ≥ 35%)(ExTRa group). The primary outcome measure was recurrence of atrial tachyarrhythmias after a 90-day blanking period postablation.
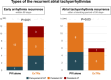
Results: Post-PVI ExTRa Mapping assessed a median of 36 sites per patient in both atria. Baseline characteristics were comparable between groups. The ExTRa group showed higher event-free survival from the primary outcome compared to the PVI alone group (85.0% vs. 67.5% at 1-year, p = 0.07). This favorable prognosis was more pronounced for patients with a large( ≥ 12 sites) area of rotational activation area (81.0% vs. 57.9% at 1-year, p = 0.01). Multivariable analysis identified the number of high %NP areas as an independent risk factor for recurrent tachyarrhythmias (HR 1.13, 95%CI 1.03-1.23, p = 0.005), while ExTRa Mapping-guided substrate ablation emerged as a unique protective factor (HR 0.38, 95%CI 0.13-0.99, p = 0.047).
Conclusion: While the reduction in atrial tachyarrhythmia recurrence of persistent AF patients did not reach statistical significance, the addition of ExTRa Mapping™-guided substrate ablation beyond PVI demonstrated promising potential, especially in patients with larger rotational activation areas.
Keywords: ExTRa mapping; atrial fibrillation; atrial fibrillation driver; catheter ablation; persistent atrial fibrillation.
© 2025 The Author(s). Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Haïssaguerre M., Jaïs P., Shah D. C., et al., “Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins,” New England Journal of Medicine 339 (1998): 659–666. - PubMed
-
- Kawaji T., Shizuta S., Morimoto T., et al., “Very Long‐Term Clinical Outcomes After Radiofrequency Catheter Ablation for Atrial Fibrillation: A Large Single‐Center Experience,” International Journal of Cardiology 249 (2017): 204–213. - PubMed
-
- Terricabras M., Piccini J. P., and Verma A., “Ablation of Persistent Atrial Fibrillation: Challenges and Solutions,” Journal of Cardiovascular Electrophysiology 31 (2020): 1809–1821. - PubMed
-
- Verma A., Jiang C., Betts T. R., et al., “Approaches to Catheter Ablation for Persistent Atrial Fibrillation,” New England Journal of Medicine 372 (2015): 1812–1822. - PubMed
-
- Konings K. T., Kirchhof C. J., Smeets J. R., Wellens H. J., Penn O. C., and Allessie M. A., “High‐Density Mapping of Electrically Induced Atrial Fibrillation in Humans,” Circulation 89 (1994): 1665–1680. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

