Cognitive control of behavior and hippocampal information processing without medial prefrontal cortex
- PMID: 40548696
- PMCID: PMC12185103
- DOI: 10.7554/eLife.104475
Cognitive control of behavior and hippocampal information processing without medial prefrontal cortex
Abstract
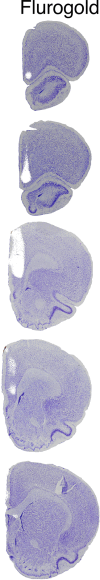
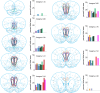
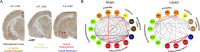
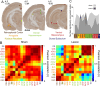
Cognitive control tasks require using one class of information while ignoring competing classes of information. The central role of the medial prefrontal cortex (mPFC) in cognitive control is well established in the primate literature and largely accepted in the rodent literature because mPFC damage causes deficits in tasks that may require cognitive control, as inferred, typically from the task design. In prior work, we used an active place avoidance task where a rat or mouse on a rotating arena is required to avoid the stationary task-relevant locations of a mild shock and ignore the rotating task-irrelevant locations of those shocks. The task is impaired by hippocampal manipulations, and the discharge of hippocampal place cell populations judiciously alternates between representing stationary locations near the shock zone and rotating locations far from the shock zone, demonstrating cognitive control concurrently in behavior and the hippocampal representation of spatial information. Here, we test whether rat mPFC lesion impairs the active place avoidance task to evaluate two competing hypotheses, a 'central-computation' hypothesis that the mPFC is essential for the computations required for cognitive control and an alternative 'local-computation' hypothesis that other brain areas can perform the computations required for cognitive control, independent of mPFC. Ibotenic acid lesion of the mPFC was effective, damaging the cingulate, prelimbic, and infralimbic cortices. The lesion also altered the normal coordination of metabolic activity across remaining structures. The lesion did not impair learning to avoid the initial location of shock or long-term place avoidance memory, but impaired avoidance after the shock was relocated. The lesion also did not impair the alternation between task-relevant and task-irrelevant hippocampal representations of place information. These findings support the local-computation hypothesis that computations required for cognitive control can occur locally in brain networks independently of the mPFC.
Keywords: decision; executive control; hippocampus; learning; memory; neural coordination; neuroscience; rat.
© 2024, Park et al.
Conflict of interest statement
EP, KO, GG, DT, KN, AA, NR, NK, SS, AF No competing interests declared
Figures


Update of
- doi: 10.1101/2019.12.20.884262
- doi: 10.7554/eLife.104475.1
- doi: 10.7554/eLife.104475.2
Similar articles
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Ventral tegmental area dopamine neural activity switches simultaneously with rule representations in the medial prefrontal cortex and hippocampus.J Neurosci. 2025 Mar 17:e1670242025. doi: 10.1523/JNEUROSCI.1670-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40097186
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Timing of Methamphetamine Exposure during Adolescence Differentially Influences Parvalbumin and Perineuronal Net Immunoreactivity in the Medial Prefrontal Cortex of Female, but Not Male, Rats.Dev Neurosci. 2025;47(1):27-39. doi: 10.1159/000538608. Epub 2024 Mar 28. Dev Neurosci. 2025. PMID: 38547851 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

