African swine fever virus MGF505-3R facilitates ferroptosis to restrict TBK1-IRF3 pathway
- PMID: 40548711
- PMCID: PMC12323604
- DOI: 10.1128/spectrum.03423-24
African swine fever virus MGF505-3R facilitates ferroptosis to restrict TBK1-IRF3 pathway
Abstract
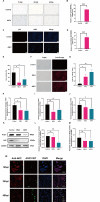
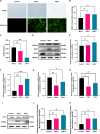
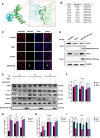
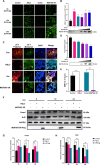
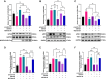
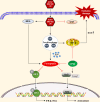
African swine fever virus (ASFV) causes hemorrhagic, severe infectious diseases and serious economic losses to the pig industry. ASFV multigene family 505 can antagonize the host's innate immunity through multiple signaling pathways and is considered an important target for vaccine development. However, the mechanism by which it induces host cell damage remains unclear. In this study, we observed that ASFV infection, similar to RSL3, can induce ferroptosis with the accumulation of reactive oxygen species (ROS) and iron, decrease glutathione peroxidase 4 (GPX4) expression, and restrain the Kelch-like ECH-associated protein 1-nuclear factor E2-related factor (Keap1-Nrf2) pathway. Moreover, the expression of ferroptosis biomarkers (LOX and PTGS2) has been moderately upregulated. Some proteins related to ASFV replication, invasion, and infection were evaluated for evidence of ferroptosis. MGF505-3R interacts with GPX4 to undergo ferroptosis, resulting in ROS accumulation, mitochondrial membrane potential destruction, and NCOA4-mediated ferritinophagy elevation. In addition, MGF505-3R suppressed the Keap1-Nrf2 pathway, while GPX4 activation counteracted its stimulatory effect on TANK-binding kinase 1 (TBK1)-IRF3 phosphorylation. Importantly, the transcription levels of interferon beta (IFN-β), ISG15, and ISG54 were elevated after GPX4 activation, suggesting that ferroptosis resistance could reverse the inhibition of the TBK1-IRF3 pathway and IFN-β levels induced by MGF505-3R. These findings provide new ideas and directions for elucidating the mechanism of ASFV-induced oxidative damage and lay a significant foundation for revealing the pathogenic mechanism of the virus by targeting ferroptosis.
Importance: We revealed that ASFV infection and MGF505-3R transfection induced the accumulation of iron and ROS, resulting in NCOA4-mediated ferritinophagy and ferroptosis, as well as restricted GPX4 expression and the Keap1-Nrf2 pathway. GPX4 activation promotes the TBK1-IRF3-IFN-β pathway and exerts antiviral activity. These findings indicate that ASFV facilitates ferroptosis, providing a proof of principle that may be applicable to oxidative damage and lipid peroxidation manipulation-based therapy for ASFV infection. Given the GPX4 downregulation in ASFV infection, GPX4 activation and ferroptosis resistance highlight its potential as a therapeutic target for viral infection.
Keywords: African swine fever virus; GPX4; IRF3; MGF505-3R; ferroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

