Active site loops of membrane-anchored metallo-β-lactamases from environmental bacteria determine cephalosporinase activity
- PMID: 40548716
- PMCID: PMC12326980
- DOI: 10.1128/aac.01918-24
Active site loops of membrane-anchored metallo-β-lactamases from environmental bacteria determine cephalosporinase activity
Abstract
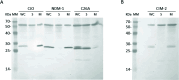
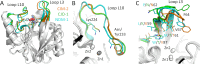
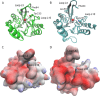
Antimicrobial resistance is a significant global public health threat that limits treatment options for bacterial infections. This situation is aggravated by the environmental spread of β-lactamase genes. In particular, metallo-β-lactamases (MBLs) hydrolyze almost all available β-lactam antibiotics, including late-generation cephalosporins and carbapenems. Among MBLs, the New Delhi metallo-β-lactamase (NDM-1) of subclass B1 has shown the most ominous dissemination. NDM variants are the only MBLs of clinical importance that are membrane-anchored, a sub-cellular localization that endows them with high stability under conditions of metal limitation. However, antibiotic resistance predates modern antibiotic usage, and environmental bacteria serve as reservoirs for resistance genes. Here, we report the biochemical and structural characterization of two membrane-bound MBLs: CJO-1 and CIM-2, from Chryseobacterium joostei and Chryseobacterium indologenes, respectively. Both MBLs confer β-lactam resistance on producer bacterial strains and hydrolyze several antibiotics, although with impaired efficiency compared to NDM-1. Crystal structures reveal differences, compared to previously studied B1 MBLs, in the active site loops and their dynamic properties that impact activity. Specifically, a hindered access to the active site with the contribution of a Tyr residue in loop L10 and the presence of a positively charged Lys residue in loop L3 limit hydrolysis of cephalosporins with charged C3 substituents. Some of these novel features are preserved in other MBLs from Chryseobacterium spp. These findings suggest that Chryseobacterium spp. could act as reservoirs of MBL genes, while informing on the diversity of structure-function relationships and dynamic behaviors within the B1 subclass of these enzymes.
Keywords: Chryseobacterium spp.; NDM; membrane-anchoring; metallo-beta-lactamase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

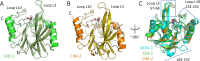
Similar articles
-
A Novel Metallo-β-Lactamase AMM-1 From Alteromonas mangrovi Reveals a Cryptic Environmental Reservoir of Carbapenem Resistance.Microb Biotechnol. 2025 Jul;18(7):e70191. doi: 10.1111/1751-7915.70191. Microb Biotechnol. 2025. PMID: 40629814 Free PMC article.
-
Mutations in ampD cause hyperproduction of AmpC and CmcB β-lactamases and high resistance to β-lactam antibiotics in Chromobacterium violaceum.Microbiol Spectr. 2025 Aug 5;13(8):e0091625. doi: 10.1128/spectrum.00916-25. Epub 2025 Jun 12. Microbiol Spectr. 2025. PMID: 40503828 Free PMC article.
-
Metallo-β-lactamase inhibitors: A continuing challenge for combating antibiotic resistance.Biophys Chem. 2024 Jun;309:107228. doi: 10.1016/j.bpc.2024.107228. Epub 2024 Mar 25. Biophys Chem. 2024. PMID: 38552402 Review.
-
Functional and structural analyses of amino acid sequence variation in PDC β-lactamase reveal different mechanistic pathways toward cefiderocol resistance in Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2025 Jul 2;69(7):e0029225. doi: 10.1128/aac.00292-25. Epub 2025 May 27. Antimicrob Agents Chemother. 2025. PMID: 40422084 Free PMC article.
-
Cefepime-taniborbactam: Ushering in the era of metallo-β-lactamase inhibition.Pharmacotherapy. 2025 Jul;45(7):448-461. doi: 10.1002/phar.70036. Epub 2025 Jun 20. Pharmacotherapy. 2025. PMID: 40542539 Review.
References
-
- WHO bacterial priority pathogens list, 2024: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. 2024 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

