Microbial diagnostic features identified across populations possess potential antitumor properties in breast cancer
- PMID: 40548719
- PMCID: PMC12282184
- DOI: 10.1128/msystems.00271-25
Microbial diagnostic features identified across populations possess potential antitumor properties in breast cancer
Abstract
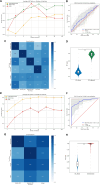
The consistency of the associations between the breast microbiome and breast cancer (BC) across various studies remains uncertain. Publicly accessible data sets from five BC studies, comprising 16S rRNA gene sequencing data from 161 BC tissues (BC_tissue), 195 BC adjacent non-cancerous tissues (BC_adjacent), and 451 normal breast tissues (normal_tissue), were retrieved from the European Nucleotide Archive. Overall, the microbial composition across the three breast tissue statuses was predominantly characterized by the phyla Proteobacteria and Firmicutes, a distribution likely attributable to the fatty acid-rich environment of the breast tissue. Comparative analysis revealed that the relative abundances of the genera Cutibacterium and Burkholderia were significantly increased in both BC_adjacent and normal_tissue compared to BC_tissue. This observation suggested a potential anticancer effect associated with these genera. Our analysis revealed a significant reduction in the abundance of Cutibacterium and Cutibacterium acnes in BC tissues, which served as specific diagnostic features for BC. This finding was corroborated by our in-house data set (n = 28), which yielded similar conclusions. Subsequent in vitro and in vivo experiments verified the potential antitumor effects of C. acnes supernatant in BC. In conclusion, our study highlighted the predictive capacity of microbial biomarkers in the onset of BC. Notably, specific bacterial species within the breast microbiome, such as Cutibacterium and C. acnes, exhibited potential as diagnostic markers for BC and may contribute significantly to antitumor activity. Nevertheless, the molecular mechanisms governing their interactions with cancer cells are not yet fully understood, necessitating further research to investigate their viability as targets for tumor prevention.IMPORTANCEAlthough a growing number of studies have highlighted the significant role of microorganisms in BC, there is a lack of consensus regarding the specific microbial genera consistently associated with breast cancer. While some studies have identified certain genera in the breast cancer environment, the results are often inconsistent and influenced by factors such as study design, population, or methodologies used. Through a comprehensive analysis of five publicly available breast cancer studies, along with validation from an in-house cohort, we found a significantly reduced abundance of Cutibacterium and C. acnes in BC tissues. In vivo and in vitro experiments demonstrated the antitumor effects of C. acnes in BC. Understanding the antitumor mechanisms of C. acnes in BC may provide potential avenues for developing novel therapeutic strategies for this disease.
Keywords: Cutibacterium acnes; antitumor properties; breast cancer; culturomics; propanoate metabolism; random forest; specific microbial feature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

