The pre-mRNA splicing modulator pladienolide B inhibits Cryptococcus neoformans germination and growth
- PMID: 40548732
- PMCID: PMC12306170
- DOI: 10.1128/msphere.00248-25
The pre-mRNA splicing modulator pladienolide B inhibits Cryptococcus neoformans germination and growth
Abstract
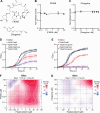
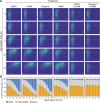
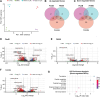
Cryptococcus neoformans is an opportunistic fungal pathogen responsible for life-threatening infections, particularly in immunocompromised individuals. The limitations of current antifungal therapies due to toxicity and the emergence of resistance highlight the need for novel treatment strategies and targets. C. neoformans has an intron-rich genome, and pre-mRNA splicing is required for expression of the vast majority of its genes. In this study, we investigated the efficacy of a human splicing inhibitor, pladienolide B (PladB), as an antifungal against C. neoformans. PladB inhibited the growth of C. neoformans in liquid culture and spore germination. The potency of PladB could be increased by simultaneous treatment with either FK506 or clorgyline. This combination treatment resulted in significant reductions in fungal growth and prevented spore germination. Transcriptomic analysis revealed that PladB inhibits splicing in C. neoformans and results in widespread intron retention. In combination with FK506, this resulted in downregulation of or intron retention in transcripts from processes vital for cellular growth, including translation, transcription, and RNA processing. Together, these results suggest that targeting RNA splicing pathways could be a promising antifungal strategy and that the effectiveness of splicing inhibitors as antifungals can be increased by co-administering drugs such as FK506.IMPORTANCEFungal infections, like those caused by Cryptococcus neoformans, can turn deadly for many patients. New treatments and therapeutic targets are needed to combat these pathogens. One potential target is the pre-mRNA processing pathway, which is required for expression of nearly all protein-coding genes in C. neoformans. We have determined that a pre-mRNA splicing inhibitor can inhibit both C. neoformans growth and germination and that the potency of this drug can be increased when used in combination with other molecules. This work provides evidence that targeting steps in pre-mRNA processing may be an effective antifungal strategy and avenue for the development of new medicines.
Keywords: C. neoformans; antifungal; inhibitor; pre-mRNA; splicing.
Conflict of interest statement
A.A.H. is a member of the scientific advisory board and carries out sponsored research for Remix Therapeutics.
Figures


Similar articles
-
Lack of an atypical PDR transporter generates an immunogenic Cryptococcus neoformans strain that drives a dysregulated and lethal immune response in murine lungs.mBio. 2025 Jul 9;16(7):e0132125. doi: 10.1128/mbio.01321-25. Epub 2025 May 30. mBio. 2025. PMID: 40444466 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The Cryptococcus neoformans transcriptome at the site of human meningitis.mBio. 2014 Feb 4;5(1):e01087-13. doi: 10.1128/mBio.01087-13. mBio. 2014. PMID: 24496797 Free PMC article.
References
-
- Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Bromley M, Brüggemann R, Garber G, Cornely OA, Gurr SJ, Harrison TS, Kuijper E, Rhodes J, Sheppard DC, Warris A, White PL, Xu J, Zwaan B, Verweij PE. 2022. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 20:557–571. doi: 10.1038/s41579-022-00720-1 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
