METTL3 regulates PRRSV replication by suppressing interferon beta through autophagy-mediated IKKε degradation
- PMID: 40548751
- PMCID: PMC12282061
- DOI: 10.1128/jvi.00098-25
METTL3 regulates PRRSV replication by suppressing interferon beta through autophagy-mediated IKKε degradation
Abstract
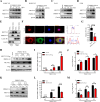
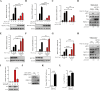
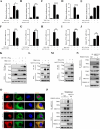
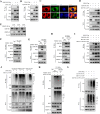
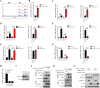
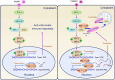
Methyltransferase-like-3 (METTL3)-mediated N6-methyladenosine (m6A) modification of messenger RNAs plays a pivotal role in regulating innate immune responses, either promoting or combating virus replication. However, the biological function of METTL3 during porcine reproductive and respiratory syndrome virus (PRRSV) infection remains unclear. In this study, we found that PRRSV infection reprograms m6A modifications in cellular transcripts, enhances METTL3 expression, and alters its subcellular distribution. Intriguingly, METTL3 overexpression facilitates PRRSV replication, whereas its deficiency suppresses it, primarily through the negative regulation of type I interferon (IFN-I) production. Further investigation revealed that METTL3 interacts with and promotes the degradation of IκB kinase-ε (IKKε) during PRRSV infection. Mechanistically, METTL3-mediated m6A modification of SQSTM1 (sequestosome 1) enhances SQSTM1 messenger RNA (mRNA) expression, increasing autophagy levels. Moreover, METTL3 facilitates the formation of K63-linked ubiquitin chains on IKKε, targeting it for degradation via SQSTM1-dependent selective autophagy. Collectively, our findings unveil a novel mechanism whereby METTL3 facilitates PRRSV replication by suppressing antiviral innate immunity, thereby offering potential targets for antiviral therapy.IMPORTANCEPorcine reproductive and respiratory syndrome (PRRS), induced by the porcine reproductive and respiratory syndrome virus (PRRSV), poses a highly contagious threat to the global swine industry, leading to substantial economic losses. The genetic variability and immune evasion capabilities of PRRSV complicate the development of effective vaccines and control strategies. Thus, a comprehensive understanding of PRRSV's immune evasion mechanisms is imperative. In this study, we reveal that METTL3 plays a pivotal role in PRRSV's evasion of interferon (IFN) immunity. Specifically, METTL3 targets IKKε, inducing its autophagy degradation and subsequently inhibiting the expression of interferon beta 1 (IFNB1). Furthermore, PRRSV infection alters the N6-methyladenosine (m6A) modification of various host genes, with notable changes observed in the m6A modification and transcriptional levels of SQSTM1, which are regulated by METTL3. This regulation is crucial for SQSTM1-mediated autophagy degradation of IKKε. Our findings offer novel insights into the mechanisms underlying host protein involvement in PRRSV's immune evasion.
Keywords: IKKε; METTL3; N6-methyladenosine (m6A); PRRSV; SQSTM1/p62; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fang P, Xie C, Pan T, Cheng T, Chen W, Xia S, Ding T, Fang J, Zhou Y, Fang L, Wei D, Xiao S. 2023. Unfolding of an RNA G-quadruplex motif in the negative strand genome of porcine reproductive and respiratory syndrome virus by host and viral helicases to promote viral replication. Nucleic Acids Res 51:10752–10767. doi: 10.1093/nar/gkad759 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

