Pentamidine inhibition of streptopain attenuates Streptococcus pyogenes virulence
- PMID: 40548752
- PMCID: PMC12323618
- DOI: 10.1128/spectrum.00758-25
Pentamidine inhibition of streptopain attenuates Streptococcus pyogenes virulence
Abstract
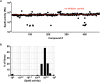
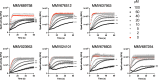
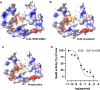
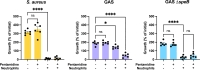
The obligate human pathogen Streptococcus pyogenes (also known as GAS; Group A Streptococcus) carries high morbidity and mortality, primarily in impoverished or resource-poor regions. The failure rate of monotherapy with conventional antibiotics is high, and invasive infections by this bacterium frequently require extensive supportive care and surgical intervention. Thus, it is important to find new compounds with adjunctive therapeutic benefits. The conserved secreted protease streptopain (Streptococcal pyogenic exotoxin B; SpeB) directly contributes to disease pathogenesis by inducing pathological inflammation, degrading tissue, and promoting the evasion of antimicrobial host defense proteins. This study screened 400 diverse off-patent drugs and drug-like compounds for inhibitors of streptopain proteolysis. Lead compounds were tested for activity at lower concentrations and anti-virulence activities during in vitro infection. Significant inhibition of streptopain was seen for pentamidine, an anti-protozoal drug approved for the treatment of Pneumocystis pneumonia, leishmaniasis, and trypanosomiasis. Streptopain inhibition rendered GAS susceptible to killing by human innate immune cells. These studies identify unexploited molecules as new starting points for drug discovery and a potential for repurposing existing drugs for the treatment of infections by GAS.IMPORTANCEStreptococcus pyogenes is a common cause of severe invasive infections. Repeated infections can trigger autoimmune diseases such as acute rheumatic fever and rheumatic heart disease. This study examines how targeting a specific, highly conserved virulence factor of the secreted cysteine protease streptopain can sensitize a serious pathogen to killing by the immune system. Manipulating the host-pathogen interaction, rather than attempting to directly kill a microbe, is a promising therapeutic strategy. Notably, its benefits include limiting off-target effects on the microbiota. Streptopain inhibitors, including the antifungal and antiparasitic drug pentamidine as identified in this work, may therefore be useful in the treatment of S. pyogenes infection.
Keywords: Streptococcus pyogenes; anti-infective; antibiotics; drug repurposing; protease; virulence factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Pentamidine inhibition of streptopain attenuates Streptococcus pyogenes virulence.bioRxiv [Preprint]. 2025 Mar 12:2025.03.12.642885. doi: 10.1101/2025.03.12.642885. bioRxiv. 2025. Update in: Microbiol Spectr. 2025 Aug 5;13(8):e0075825. doi: 10.1128/spectrum.00758-25. PMID: 40161583 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

