Elucidating the Curtin-Hammett Principle in Glycosylation Reactions: The Decisive Role of Equatorial Glycosyl Triflates
- PMID: 40548839
- PMCID: PMC12232320
- DOI: 10.1021/jacs.5c03519
Elucidating the Curtin-Hammett Principle in Glycosylation Reactions: The Decisive Role of Equatorial Glycosyl Triflates
Abstract
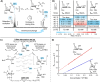
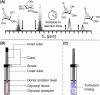
The glycosylation reaction represents a crucial and challenging reaction used in oligosaccharide synthesis. Specifically, attaining complete stereocontrol during glycosylation reactions remains challenging. Its complex nature is defined by the highly reactive intermediates that form upon the activation of a glycosyl donor. Low-abundant species may afford the major product via Curtin-Hammett kinetics and have long been proposed to play a major role. Therefore, characterizing these elusive stereodirecting intermediates is key to understanding glycosylation reaction mechanisms. Herein, we applied a combination of (exchange) NMR techniques to establish the equilibration rates of glycosyl triflate reaction intermediates and their ensuing glycosylation reaction kinetics. To this end, we studied the glycosylation reactions of 6,3-mannuronic acid and 6,3-glucuronic acid lactone donors. Using the complete set of reaction kinetics data, we constructed a computational kinetic model that shows that these compounds indeed react according to a Curtin-Hammett scenario. Furthermore, we were able to rationalize the observed stereochemical reaction outcomes using quantum-chemically computed potential energy surfaces for these glycosylation reactions. Hence, this workflow can now be used to obtain a complete reaction kinetics overview to retrieve the reaction pathway(s) that drive product formation.
Figures





References
-
- Crich D., Sun S.. Are glycosyl triflates intermediates in the sulfoxide glycosylation method? A chemical and 1H, 13C, and 19F NMR spectroscopic investigation. J. Am. Chem. Soc. 1997;119(46):11217–11223. doi: 10.1021/ja971239r. - DOI
-
- Seeman J. I.. Effect of conformational change on reactivity in organic chemistry. Evaluations, applications, and extensions of Curtin-Hammett Winstein-Holness kinetics. Chem. Rev. 1983;83(2):83–134. doi: 10.1021/cr00054a001. - DOI
-
- Seeman J. I.. The Curtin-Hammett principle and the Winstein-Holness equation: new definition and recent extensions to classical concepts. J. Chem. Educ. 1986;63(1):42. doi: 10.1021/ed063p42. - DOI
LinkOut - more resources
Full Text Sources

