A four-in-one replicase integrating key enzymatic activities for DNA replication
- PMID: 40548937
- PMCID: PMC12205980
- DOI: 10.1093/nar/gkaf542
A four-in-one replicase integrating key enzymatic activities for DNA replication
Abstract
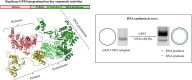
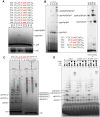
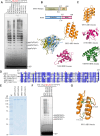
DNA replication is a fundamental process in all living organisms. As the most diverse and abundant biological entities on Earth, bacteriophages may utilize unconventional methods for genome replication. In this study, we identified a novel DNA replicase, GP55, from lactococcal phage 1706. GP55 comprises a helicase domain, a distinctive archaeo-eukaryotic primase domain, and a family B DNA polymerase domain, collectively exhibiting helicase, primase, and DNA polymerase activities, along with intrinsic 3'-5' exonuclease activity. Notably, the helicase activity of GP55 is UTP/dTTP-dependent rather than ATP-dependent and facilitates strand displacement during DNA synthesis. GP55 exhibits a unique primase activity, recognizing specific but less stringent DNA sequences and preferring GTP for the initiation of RNA primer synthesis. Additionally, a newly identified α-helix domain, composed of two pairs of parallel α-helices, was found to be essential for its primase activity. The multiple activities enable GP55 to efficiently synthesize DNA de novo in the presence of dNTPs and NTPs. This study reveals a concise strategy employed by bacteriophages for genome replication using multifunctional replicases.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

