Interplay between Nrf2 and ROS in regulating epithelial-mesenchymal transition: implications for cancer metastasis and therapy
- PMID: 40549063
- PMCID: PMC12185618
- DOI: 10.1007/s11033-025-10731-9
Interplay between Nrf2 and ROS in regulating epithelial-mesenchymal transition: implications for cancer metastasis and therapy
Abstract
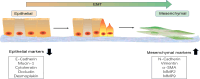
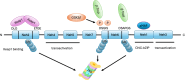
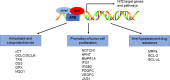
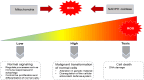
Epithelial-mesenchymal transition (EMT), including developmental (Type I), wound healing (Type II), and pathological (Type III) subtypes, constitutes a critical driver of cancer metastasis. This review analyzes the redox interplay between nuclear factor erythroid 2-related factor 2 (Nrf2) and reactive oxygen species (ROS) in EMT regulation and cancer progression. Nrf2 maintains redox homeostasis through antioxidant gene activation while paradoxically promoting tumor survival and drug resistance via Keap1-dependent degradation and phosphorylation-mediated stabilization. ROS generated through mitochondrial and NADPH oxidase pathways exhibit dual functionality: moderate levels activate EMT transcription factors to drive metastasis and cancer stem cells (CSCs) plasticity, whereas excessive ROS induce apoptosis and ferroptosis. While Nrf2 typically suppresses EMT through ROS neutralization and epithelial integrity preservation, chronic Nrf2 activation in CSCs paradoxically sustains metastatic potential through redox buffering. This synthesis delineates the spatiotemporal regulation of Nrf2-ROS-EMT networks across tumor microenvironments, emphasizing therapeutic opportunities through redox balance modulation and pathway-specific Nrf2 inhibition in advanced malignancies.
Keywords: Cancer stem cells; Epithelial-mesenchymal transition (EMT); Nrf2; Oxidative stress; ROS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Targeting Drp1 inhibits ESCC progression via the ROS-PGC1-α-Nrf1/2 pathway.J Transl Med. 2025 Jun 17;23(1):674. doi: 10.1186/s12967-025-06697-8. J Transl Med. 2025. PMID: 40528181 Free PMC article.
-
Elevated level of mitochondrial reactive oxygen species via fatty acid β-oxidation in cancer stem cells promotes cancer metastasis by inducing epithelial-mesenchymal transition.Stem Cell Res Ther. 2019 Jun 13;10(1):175. doi: 10.1186/s13287-019-1265-2. Stem Cell Res Ther. 2019. PMID: 31196164 Free PMC article.
-
MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma.J Exp Clin Cancer Res. 2019 Mar 25;38(1):136. doi: 10.1186/s13046-019-1135-x. J Exp Clin Cancer Res. 2019. PMID: 30909929 Free PMC article.
-
Crosstalk between NRF2 and Dicer through metastasis regulating MicroRNAs; mir-34a, mir-200 family and mir-103/107 family.Arch Biochem Biophys. 2020 Jun 15;686:108326. doi: 10.1016/j.abb.2020.108326. Epub 2020 Mar 3. Arch Biochem Biophys. 2020. PMID: 32142889 Review.
-
The role of nuclear factor erythroid 2-related factor 2 (NRF2) in normal and pathological pregnancy: A systematic review.Am J Reprod Immunol. 2021 Dec;86(6):e13496. doi: 10.1111/aji.13496. Epub 2021 Sep 19. Am J Reprod Immunol. 2021. PMID: 34467607
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48. 10.3322/caac.21763 - PubMed
-
- Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, Zhou Z, Yin P, Zhou M (2023) National and subnational trends in cancer burden in china, 2005-20: an analysis of National mortality surveillance data. Lancet Public Health 8(12):e943–e955. 10.1016/S2468-2667(23)00211-6 - PubMed
-
- Pastushenko I, Blanpain C (2019) EMT transition States during tumor progression and metastasis. Trends Cell Biol 29(3):212–226. 10.1016/j.tcb.2018.12.001 - PubMed
-
- Gundamaraju R, Lu W, Paul MK, Jha NK, Gupta PK, Ojha S, Chattopadhyay I, Rao PV, Ghavami S (2022) Autophagy and EMT in cancer and metastasis: who controls whom? Biochim Biophys Acta Mol Basis Dis 1868(9):166431. 10.1016/j.bbadis.2022.166431 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

