Cost-Effectiveness of Trimodal Therapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer
- PMID: 40549384
- PMCID: PMC12186510
- DOI: 10.1001/jamanetworkopen.2025.17056
Cost-Effectiveness of Trimodal Therapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer
Abstract
Importance: Trimodal therapy (TMT) is included as an alternative to radical cystectomy (RC) for definitive management of muscle-invasive bladder cancer (MIBC) in current clinical guidelines. Moreover, a 2023 retrospective analysis reported similar oncologic outcomes between these treatments among patients deemed fit for RC. Data regarding the comparative value of these treatments are lacking.
Objective: To evaluate the comparative cost-effectiveness of TMT and RC for treatment of MIBC.
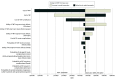
Design, setting, and participants: This economic evaluation compared cost-effectiveness of treatments using a health transition state microsimulation model of patients with bladder cancer treated between 2005 and 2017 with 5- and 10-year horizons from a Medicare perspective. Model probabilities were informed by multicenter retrospective analyses published in 2023 comparing TMT with RC. The index patient was aged 71 years, with clinical stage T2-4aN0M0 MIBC, solitary tumor smaller than 7 cm, no or unilateral hydronephrosis, adequate bladder function, and lack of multifocal or extensive carcinoma in situ. Patients unfit for RC, radiation, or cisplatin-based chemotherapy were excluded.
Exposures: TMT and RC.
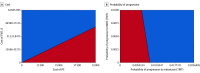
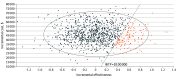
Main outcome and measures: Primary outcomes included effectiveness measured in quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICER) using a willingness-to-pay threshold of $100 000 per QALY. Sensitivity analyses were performed to assess the robustness of the model.
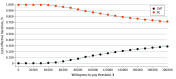
Results: For the index patient, at 5 years, the average cost was $30 525 higher for TMT than RC. Average QALYs were 3.87 and 3.94 for RC and TMT, respectively. As such, TMT was not cost-effective at 5-year (ICER, $464 291 per QALY) or 10-year (ICER, $308 638 per QALY) time horizons. On 1-way sensitivity analyses, TMT would become cost-effective if (1) TMT costs were less than $17 605; or (2) TMT resulted in an 11.6% improvement in metastasis-free survival relative to RC.
Conclusions and relevance: In this economic evaluation study of TMT and RC for treatment of MIBC, TMT was associated with improved quality of life but was not cost-effective relative to RC at 5 and 10 years given higher treatment costs. These findings highlight the importance of developing policy initiatives that help reduce TMT costs and of providing patients with accurate expectations of long-term toxic effects to help guide preference-sensitive care.
Conflict of interest statement
Figures
References
-
- National Comprehensive Cancer Network . Clinical practice guidelines in oncology for bladder cancer version 4.2024. Accessed September 20, 2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1417
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

