Development of Dual 'RT-LAMP-LFA' Rapid Detection Technology With Gold Magnetic Nanoparticles for Influenza Virus
- PMID: 40549481
- PMCID: PMC12184558
- DOI: 10.1111/1751-7915.70169
Development of Dual 'RT-LAMP-LFA' Rapid Detection Technology With Gold Magnetic Nanoparticles for Influenza Virus
Abstract
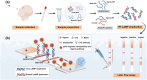
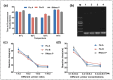
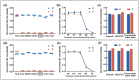
Seasonal and persistent outbreaks of influenza viruses represent a significant challenge to global public health. Rapid, convenient and accurate diagnosis methods of influenza viruses are crucial for timely treatment to mitigate morbidity and mortality during both seasonal epidemics and pandemics. However, current diagnostic tools often face limitations in speed, accuracy or complexity of result interpretation; there is a great need for more efficient detection technology for influenza virus, especially for use in resource-limited settings or during large-scale outbreaks. This study developed a dual 'RT-LAMP-LFA' detection technology with gold magnetic nanoparticles for influenza virus. This method can simultaneously detect influenza A and B genes as well as internal reference genes within 35 min, with a detection limit of 80 copies/mL. This is the first time the RNase P gene has been introduced into a gold magnetic nanoparticle lateral flow assay system as a quality control measure to monitor the entire sampling and amplification process in virus detection and reveals the effects of loop primer deficiencies on the stability of the dual 'RT-LAMP-LFA' detection technology. Using fluorescent PCR detection technology as a benchmark, the analysis of a total of 70 clinical samples demonstrated a 100% agreement rate, confirming the applicability and accuracy of the dual 'RT-LAMP-LFA' detection system. This dual 'RT-LAMP-LFA' detection technology offers a novel option for diagnostic technology in hierarchical medical testing, presenting significant social importance and broad application prospects.
Keywords: dual loop‐mediated isothermal amplification; gold magnetic nanoparticles; influenza virus; lateral flow assay.
© 2025 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Aonuma, H. , Yoshimura A., Kobayashi T., et al. 2010. “A Single Fluorescence‐Based LAMP Reaction for Identifying Multiple Parasites in Mosquitoes.” Experimental Parasitology 125: 179–183. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

