Clinical efficacy and Safety of Baloxavir Marboxil compared with Oseltamivir against influenza virus in children: A systematic review and meta-analysis
- PMID: 40549724
- PMCID: PMC12185026
- DOI: 10.1371/journal.pone.0326777
Clinical efficacy and Safety of Baloxavir Marboxil compared with Oseltamivir against influenza virus in children: A systematic review and meta-analysis
Abstract
Objective: Comparing the clinical efficacy and safety of baloxavir marboxil and oseltamivir against influenza viruses in children, to provide theoretical references for clinical practice.
Methods: A systematic search of PubMed, Embase, Web of Science, Cochrane Library, Epistemonikos, CNKI, Wipu.com, Wan Fang Database, and China Biomedical Literature Database for articles published up to December 25th, 2024, was conducted. After literature screening, data extraction, and quality evaluation, descriptive analysis was performed.
Results: Eight papers were included, comprising three randomized controlled studies and Five cohort studies, involving 3141 patients (1745 in the baloxavir marboxil group and 1396 in the oseltamivir group). Meta-analysis revealed no significant differences in time to remission of influenza symptoms and duration of fever between the two groups. However, baloxavir marboxil demonstrated a significantly greater reduction in influenza virus titer and RNA load. Additionally, the incidence of adverse events was significantly lower with baloxavir marboxil (p = 0.03).
Conclusions: Baloxavir marboxil appears more effective than oseltamivir in reducing viral load and is associated with fewer adverse events in children with influenza, while both drugs yield comparable effects in relieving symptoms. Given the limited number of included studies and absence of subgroup analyses, further well-designed trials are needed to corroborate these findings. PROSPERO Registration Number: CRD42024565338.
Copyright: © 2025 Zhu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


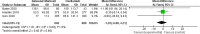
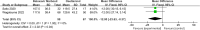
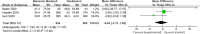
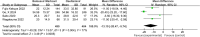
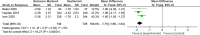
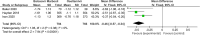
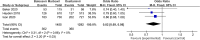

References
-
- Govorkova EA, Takashita E, Daniels RS, Fujisaki S, Presser LD, Patel MC, et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018-2020. Antiviral Res. 2022;200:105281. doi: 10.1016/j.antiviral.2022.105281 - DOI - PMC - PubMed
-
- Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022-23 Influenza Season. MMWR Recomm Rep. 2022;71(1):1–28. doi: 10.15585/mmwr.rr7101a1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

