Understanding PET Hydrolysis via Reactive Molecular Dynamics Simulation and Experimental Investigation
- PMID: 40549878
- PMCID: PMC12235640
- DOI: 10.1021/acs.jpcb.5c03080
Understanding PET Hydrolysis via Reactive Molecular Dynamics Simulation and Experimental Investigation
Abstract
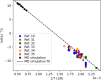
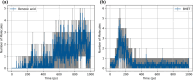
Polyethylene terephthalate (PET), a widely used polymer in packaging applications, has posed significant environmental challenges due to its resistance to environmental degradation. Chemical recycling via hydrolysis offers a circular solution by breaking PET down into its monomers, terephthalic acid and ethylene glycol, which can then be repolymerized into new PET. Despite its promise, the detailed pathways of PET hydrolysis─particularly the interplay between hydrolysis and thermal degradation─remain a topic of scientific debate. We combine reactive molecular dynamics (MD) simulations with experimental studies to elucidate key reaction pathways, intermediate species, and the temperature-dependent evolution of degradation products. Molecular dynamics simulations offer detailed insights into molecular motions and interactions that are often elusive in experimental setups, thus enhancing our understanding of the complex dynamics at play during PET decomposition. By systematically examining bond dissociation, intermediate species, and product formation at various temperatures, this study elucidates how hydrolysis and thermal degradation pathways evolve and interact. Furthermore, a severity index approach is employed to directly compare TPA yields from simulations with corresponding experimental data.
Figures






Similar articles
-
A New Measure of Quantified Social Health Is Associated With Levels of Discomfort, Capability, and Mental and General Health Among Patients Seeking Musculoskeletal Specialty Care.Clin Orthop Relat Res. 2025 Apr 1;483(4):647-663. doi: 10.1097/CORR.0000000000003394. Epub 2025 Feb 5. Clin Orthop Relat Res. 2025. PMID: 39915110
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
How Can the Environmental Impact of Orthopaedic Surgery Be Measured and Reduced? Using Anterior Cruciate Ligament Reconstruction as a Test Case.Clin Orthop Relat Res. 2025 Jan 1;483(1):7-19. doi: 10.1097/CORR.0000000000003242. Clin Orthop Relat Res. 2025. PMID: 39660662
References
-
- Nisticò R.. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020;90:106707. doi: 10.1016/j.polymertesting.2020.106707. - DOI
-
- U.S. Environmental Protection Agency (EPA) . Impacts of Plastic Pollution. https://www.epa.gov/plastics/impacts-plastic-pollution (accessed Apr 17, 2025).
-
- Barnard E., Rubio Arias J. J., Thielemans W., Thielemans W.. Chemolytic Depolymerisation of PET: A Review. Green Chem. 2021;23:3765–3789. doi: 10.1039/D1GC00887K. - DOI
-
- Romão W., Spinacé M. A. S., Paoli M. A. D.. Poly(Ethylene Terephthalate), PET: A Review on the Synthesis Processes, Degradation Mechanisms and Its Recycling. Polímeros. 2009;19(2):121–132. doi: 10.1590/S0104-14282009000200009. - DOI
LinkOut - more resources
Full Text Sources

