Molecular contacts in self-assembling clusters of membrane proteins
- PMID: 40549920
- PMCID: PMC12232663
- DOI: 10.1073/pnas.2507112122
Molecular contacts in self-assembling clusters of membrane proteins
Abstract
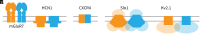
Motivated by recent data pointing to the existence of homo-oligomeric assemblies of membrane proteins called higher-order transient structures, and their apparent role in connecting components of membrane signal pathways, we examine here by cryoelectron microscopy some of the protein-protein interactions that occur in cluster formation. Metabotropic glutamate receptors and HCN ion channels inside clusters contact their neighbors through structured extracellular and intracellular domains, respectively. Other ion channels, including Kv2.1 and Slo1, appear to form clusters through prominent intrinsically disordered sequences in the cytoplasm. These distinct modes of interaction are associated with clusters exhibiting varying degrees of compactness and order. We conclude that nature utilizes a variety of ways to form connections between membrane proteins in self-assembled clusters.
Keywords: HOTS; clustering; cryo-EM; membrane proteins.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





Similar articles
-
Evidence-based toxicology: a comprehensive framework for causation.Hum Exp Toxicol. 2005 Apr;24(4):161-201. doi: 10.1191/0960327105ht517oa. Hum Exp Toxicol. 2005. PMID: 15957536
-
Adenovirus Modulates Toll-Like Receptor 4 Signaling by Reprogramming ORP1L-VAP Protein Contacts for Cholesterol Transport from Endosomes to the Endoplasmic Reticulum.J Virol. 2017 Feb 28;91(6):e01904-16. doi: 10.1128/JVI.01904-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077646 Free PMC article.
-
Murine norovirus allosteric escape mutants mimic gut activation.J Virol. 2025 Jun 17;99(6):e0021925. doi: 10.1128/jvi.00219-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353669 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
Heterodimers Revolutionize the Field of Metabotropic Glutamate Receptors.Neuroscience. 2025 Jul 10;578:46-58. doi: 10.1016/j.neuroscience.2024.06.013. Epub 2024 Jun 25. Neuroscience. 2025. PMID: 38936459 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

