Evaluation of a Novel eHealth Tool for Pulmonary Rehabilitation in People With Chronic Obstructive Pulmonary Disease: Randomized Controlled Pilot and Feasibility Trial
- PMID: 40550122
- PMCID: PMC12235207
- DOI: 10.2196/68195
Evaluation of a Novel eHealth Tool for Pulmonary Rehabilitation in People With Chronic Obstructive Pulmonary Disease: Randomized Controlled Pilot and Feasibility Trial
Abstract
Background: There is a growing interest in eHealth solutions to enhance access to and use of pulmonary rehabilitation for people with chronic obstructive pulmonary disease (COPD).
Objective: This study aims to evaluate the feasibility of a novel eHealth tool (Me&COPD) to support pulmonary rehabilitation concerning usability, exercise adherence, intensity, progression, and adverse events. Moreover, this study aims to evaluate clinical outcome measures to prepare for a future larger trial.
Methods: A multicenter, parallel-group randomized controlled pilot and feasibility trial was conducted in 6 primary health care centers. People with mild to severe COPD were recruited by physiotherapists at the included health care centers and randomized either to the intervention group with access to Me&COPD for 3 months or to the control group receiving usual care. The Me&COPD tool comprised audio-visual and written self-management strategies, including an individually tailored home-based exercise program and interaction with a physiotherapist. The exercise program was prescribed in a face-to-face meeting with a physiotherapist, and thereafter it was regularly reviewed and adjusted through the eHealth tool. The primary outcome, usability, was self-assessed at intervention completion in the intervention group and among participating physiotherapists (n=7) using the Swedish version of the Mobile Health App Usability Questionnaire (S-MAUQ). In addition, use data on exercise adherence, intensity, and progression and adverse events were exported from the eHealth tool. Clinical outcomes, assessed by blinded assessors at baseline and 3 months in the intervention and control groups, included exercise capacity, balance, physical activity level, COPD-related symptoms, and health-related quality of life. Descriptive statistics were used for analysis.
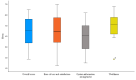
Results: In total, 22 participants (women: n=12, 55%), aged 72.3 (SD 8.4) years on average, were included in the intervention (n=15) and control (n=7) groups. The mean overall S-MAUQ scores out of 7 (highest possible usability) were 4.4 (SD 1.5) for participants and 4.5 (SD 1.2) for physiotherapists. Among the subscales, the highest score was assigned to usefulness among participants (S-MAUQ: mean 4.9, SD 1.3) and physiotherapists (S-MAUQ: mean 5.1, SD 1.7). No severe adverse events were registered, although exercise adherence, intensity, and progression evaluation were limited by incomplete exercise session registration. The test procedures and the clinical outcome measures used were found to be feasible for the participants and the assessors.
Conclusions: The novel eHealth tool, Me&COPD, seemed feasible in terms of safety and had acceptable usability among people with COPD and participating physiotherapists. Usability may be improved by better organization of the information and simplification of the exercise diary to enable collection of data on exercise adherence, intensity, and progression through the eHealth tool. The test procedures seemed feasible, although the recruitment process needs further consideration. The effectiveness of the intervention remains to be evaluated in a future larger trial.
Trial registration: ClinicalTrials.gov NCT05086341; https://clinicaltrials.gov/study/NCT05086341.
Keywords: COPD; RCT; chronic obstructive pulmonary disease; eHealth; eHealth tool; feasibility trial; pulmonary rehabilitation; randomized controlled trial; telerehabilitation; usability testing; user-centered design.
©Åsa Karlsson, Pernilla Sönnerfors, Sara Lundell, Annika Toots, Karin Wadell. Originally published in JMIR Formative Research (https://formative.jmir.org), 23.06.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015 Feb 23;2015(2):CD003793. doi: 10.1002/14651858.CD003793.pub3. https://europepmc.org/abstract/MED/25705944 - DOI - PMC - PubMed
-
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, Pitta F, Sewell L, Raskin J, Bourbeau J, Crouch R, Franssen FM, Casaburi R, Vercoulen JH, Vogiatzis I, Gosselink R, Clini EM, Effing TW, Maltais F, van der Palen J, Troosters T, Janssen DJ, Collins E, Garcia-Aymerich J, Brooks D, Fahy BF, Puhan MA, Hoogendoorn M, Garrod R, Schols AM, Carlin B, Benzo R, Meek P, Morgan M, Rutten-van Mölken MP, Ries AL, Make B, Goldstein RS, Dowson CA, Brozek JL, Donner CF, Wouters EF. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013 Oct 15;188(8):e13–64. doi: 10.1164/rccm.201309-1634ST. - DOI - PubMed
-
- Nationella riktlinjer för vård vid astma och KOL: stöd för styrning och ledning. Socialstyrelsen. 2020. Dec, [2025-06-05]. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelk... .
-
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, Spruit MA, Masefield S, Casaburi R, Clini EM, Crouch R, Garcia-Aymerich J, Garvey C, Goldstein RS, Hill K, Morgan M, Nici L, Pitta F, Ries AL, Singh SJ, Troosters T, Wijkstra PJ, Yawn BP, ZuWallack RL. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015 Dec 01;192(11):1373–86. doi: 10.1164/rccm.201510-1966ST. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

