Clinically directed initiation versus routine use of amoxicillin-clavulanate and the risk of local complications among patients with haemotoxic snakebite envenomation treated at a teaching hospital in southern India: a randomised, non-inferiority trial
- PMID: 40550712
- PMCID: PMC12186035
- DOI: 10.1136/bmjopen-2024-094409
Clinically directed initiation versus routine use of amoxicillin-clavulanate and the risk of local complications among patients with haemotoxic snakebite envenomation treated at a teaching hospital in southern India: a randomised, non-inferiority trial
Abstract
Objective: Amoxicillin-clavulanate is commonly used to prevent infections following snakebites despite the lack of clinical evidence. We aimed to demonstrate that clinically directed initiation of amoxicillin-clavulanate would be non-inferior to routine use in this setting.
Design: Open-label, randomised, non-inferiority trial with blinded adjudication of endpoints.
Setting: Emergency department of a teaching hospital in southern India.
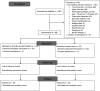
Participants: Adults with local swelling following snakebites within 24 hours of bite.
Interventions: In the routine use strategy, intravenous followed by oral amoxicillin-clavulanate was administered for at least 5 days. In the clinically directed strategy, the antibiotic was only initiated for clinical failures.
Primary and secondary outcome measures: Primary outcomes were protocol-defined clinical failure and total antibiotic consumption. Non-inferiority margin was prespecified as 10%. Secondary outcomes were the length of hospital stay, total antivenom consumption, new-onset organ failure, bleeding requiring transfusion, death/need for surgical intervention and drug-related adverse events.
Results: The trial was prematurely stopped due to the COVID-19 situation after randomising 66 patients-34 to clinically directed initiation and 32 to routine use arms. Russell's viper was the most common (21 (32%)) biting snake species identified; 52 (79%) patients had evidence of haemotoxic envenomation at baseline, and 24 (36%) patients developed AKI. There were 10 clinical failures-six in the clinically directed initiation arm and four in the routine use arm. The difference in clinical failure between the two arms was 5.2% (-12.0%-21.7%; p=0.291); the upper bound of the CI exceeded the prespecified non-inferiority margin. Total antibiotic consumption, expressed in DDDs, was significantly lower in the clinically directed initiation arm (0 (0-1) vs 5.31 (4.67-6.17); p<0.001). Three serious adverse events resulting in two deaths (one in each arm) were observed.
Conclusions: We could not demonstrate the non-inferiority of clinically directed initiation compared with routine use of amoxicillin-clavulanate among patients with local swelling caused by haemotoxic snakebites. However, the frequency of clinical failures was similar, and antibiotic consumption was substantially lower with the clinically directed initiation strategy.
Trial registration number: ClinicalTrials.gov; NCT02570347.
Keywords: Randomized Controlled Trial; TOXICOLOGY; Tropical medicine.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Interventions for necrotizing soft tissue infections in adults.Cochrane Database Syst Rev. 2018 May 31;5(5):CD011680. doi: 10.1002/14651858.CD011680.pub2. Cochrane Database Syst Rev. 2018. PMID: 29851032 Free PMC article.
-
Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation.Cochrane Database Syst Rev. 2012 May 16;2012(5):CD004269. doi: 10.1002/14651858.CD004269.pub3. Cochrane Database Syst Rev. 2012. PMID: 22592695 Free PMC article.
-
Interventions for children with ear discharge occurring at least two weeks following grommet (ventilation tube) insertion.Cochrane Database Syst Rev. 2016 Nov 17;11(11):CD011684. doi: 10.1002/14651858.CD011684.pub2. Cochrane Database Syst Rev. 2016. PMID: 27854381 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Systemic antibiotics for chronic suppurative otitis media.Cochrane Database Syst Rev. 2021 Feb 4;2(2):CD013052. doi: 10.1002/14651858.CD013052.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2025 Jun 9;6:CD013052. doi: 10.1002/14651858.CD013052.pub3. PMID: 35819801 Free PMC article. Updated.
References
-
- Jorge MT, Malaque C, Ribeiro LA, et al. Failure of chloramphenicol prophylaxis to reduce the frequency of abscess formation as a complication of envenoming by Bothrops snakes in Brazil: a double-blind randomized controlled trial. Trans R Soc Trop Med Hyg. 2004;98:529–34. doi: 10.1016/j.trstmh.2003.12.009. - DOI - PubMed
-
- Warrell AD. Guidelines for management of snakebites. New Delhi: WHO Regional Office South East Asia; 2010.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
