New Generation Modified Azole Antifungals against Multidrug-Resistant Candida auris
- PMID: 40550788
- PMCID: PMC12257509
- DOI: 10.1021/acs.jmedchem.5c01253
New Generation Modified Azole Antifungals against Multidrug-Resistant Candida auris
Abstract
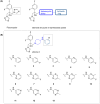
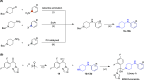
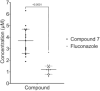
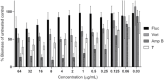
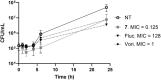
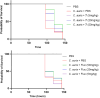
The rise of antifungal resistance and limited treatment options highlight the urgent need for new drug classes. Candida auris is a serious global health threat with few effective therapies. In this study, novel azole-based compounds were developed by modifying the azole core with cyclic heteroaliphatic linkers connecting aromatic and heteroaromatic rings. Several compounds showed potent activity against C. auris, including azole-resistant strains, with MICs ranging from 0.016 to 4 μg/mL. The compounds also demonstrated strong activity against C. albicans, Nakaseomyces glabratus, C. tropicalis, and C. parapsilosis, with MICs mostly below 1 μg/mL. Compounds 7, 18, and 21 were more potent than fluconazole. Compound 7 inhibited CYP51, eradicated C. auris biofilms, and showed better intracellular accumulation than fluconazole. In vivo studies in Galleria mellonella and Drosophila melanogaster confirmed efficacy at 5 mg/kg and no toxicity up to 50 mg/kg, supporting further development of this scaffold against multidrug-resistant C. auris infections.
Figures


References
-
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED); U.S. Department of Health & Human Services, https://www.cdc.gov/fungal/antimicrobial-resistant-fungi/. (Accessed 02 June 2025).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

