PRDM16 acts as a homeostasis regulation factor to suppress the transition of AKI to CKD via upregulation of eukaryotic initiation factor 6
- PMID: 40551013
- PMCID: PMC12185847
- DOI: 10.1007/s00018-025-05766-x
PRDM16 acts as a homeostasis regulation factor to suppress the transition of AKI to CKD via upregulation of eukaryotic initiation factor 6
Abstract
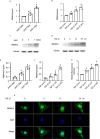
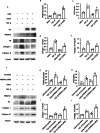
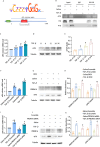
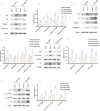
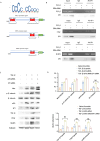
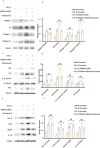
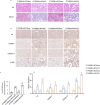
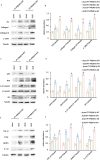
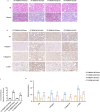
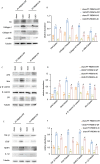
Progressing from acute kidney injury (AKI) to chronic kidney disease (CKD) is acknowledged as a significant clinical challenge. Our recent works indicated that PR domain-containing 16 (PRDM16) impedes the progression of AKI and DKD. Nonetheless, the specific function and regulatory mechanism of PRDM16 during the AKI to CKD transition remain incompletely understood. In this investigation, it was identified that PRDM16 mitigates TGF-β1-induced renal tubulointerstitial fibrosis in BUMPT cells. From a mechanistic perspective, PRDM16 was found to enhance the expression of eif6, which subsequently suppressed TGF-β, CTGF, and NLRP3 levels via the suppression of the Wnt/β-catenin/SP1 signaling cascade. Additionally, knock-in of PRDM16 in kidney proximal tubules resulted in increased expression of eIF6, thereby restraining the stimulation of the Wnt/β-catenin/SP1 pathway, reducing the production of TGF-β, CTGF, and NLRP3, and consequently limiting renal tubulointerstitial fibrosis progression in both unilateral ureteral obstruction and ischemia-reperfusion-injury mouse models.Moreover, overexpression of PRDM16 following ischemia-induced AKI was shown to attenuate renal tubulointerstitial fibrosis and the eIF6/Wnt/β-catenin/SP1/TGF-β, CTGF, and NLRP3 axis. Finally, the PRDM16/eIF6/Wnt/β-catenin/SP1/TGF-β, CTGF, and NLRP3 axis were analyzed in renal biopsies from individuals with minimal change disease and severe obstructive nephropathy. Collectively, these findings indicate that PRDM16-mediated eIF6 induction serves to impede the transition from AKI to CKD by suppressing the Wnt/β-catenin/SP1/TGF-β, CTGF, and NLRP3 axis.
Keywords: AKI; CKD; PRDM16; eIF6.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The researchers affirm the absence of any competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

