Closed-loop electrical stimulation prevents focal epilepsy progression and long-term memory impairment
- PMID: 40551024
- PMCID: PMC12321579
- DOI: 10.1038/s41593-025-01988-1
Closed-loop electrical stimulation prevents focal epilepsy progression and long-term memory impairment
Abstract
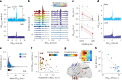
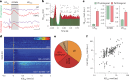
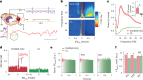
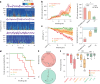
Interictal epileptiform discharges (IEDs) are expressed in epileptic networks and disrupt cognitive functions. It is unclear whether addressing IED-induced dysfunction could improve epilepsy outcomes, as most therapeutic approaches target seizures. We show, in a kindling model of progressive focal epilepsy, that IEDs produce pathological oscillatory coupling associated with prolonged, hypersynchronous neural spiking in synaptically connected cortex and expand the brain territory capable of generating IEDs. A similar relationship between IED-mediated oscillatory coupling and temporal organization of IEDs across brain regions was identified in human participants with refractory focal epilepsy. Spatiotemporally targeted closed-loop electrical stimulation triggered on hippocampal IED occurrence eliminated the abnormal cortical activity patterns, preventing the spread of the epileptic network and ameliorating long-term spatial memory deficits in rodents. These findings suggest that stimulation-based network interventions that normalize interictal dynamics may be an effective treatment of epilepsy and its comorbidities, with a low barrier to clinical translation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Update of
-
Closed-loop electrical stimulation to prevent focal epilepsy progression and long-term memory impairment.bioRxiv [Preprint]. 2024 Feb 12:2024.02.09.579660. doi: 10.1101/2024.02.09.579660. bioRxiv. 2024. Update in: Nat Neurosci. 2025 Aug;28(8):1753-1762. doi: 10.1038/s41593-025-01988-1. PMID: 38405990 Free PMC article. Updated. Preprint.
References
-
- Lagarde, S. et al. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain141, 2966–2980 (2018). - PubMed
-
- Hermann, B. P. et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann. Neurol.60, 80–87 (2006). - PubMed
-
- Kwan, P. & Brodie, M. J. Early identification of refractory epilepsy. N. Engl. J. Med.342, 314–319 (2000). - PubMed
-
- Perrine, K. et al. The relationship of neuropsychological functioning to quality of life in epilepsy. Arch. Neurol.52, 997–1003 (1995). - PubMed
-
- Boylan, L. S. et al. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology62, 258–261 (2004). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

