The soluble epoxide hydrolase inhibitor TPPU alleviates Aβ-mediated neuroinflammatory responses in Drosophila melanogaster and cellular models of alzheimer's disease
- PMID: 40551105
- PMCID: PMC12183823
- DOI: 10.1186/s12950-025-00449-7
The soluble epoxide hydrolase inhibitor TPPU alleviates Aβ-mediated neuroinflammatory responses in Drosophila melanogaster and cellular models of alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) is a common neurodegenerative disease, and its pathogenesis is closely associated with neuroinflammation. The control of neuroinflammation in AD is the focus of current research. soluble epoxide hydrolase (sEH) protein is increased in the brain tissues of patients with AD and has been targeted by multiple genome wide association studies as a prime target for treating AD. Since sEH induces nerve inflammation by degrading epoxyeicosatrienoic acids (EETs), application of sEH inhibitor and sEH gene knockout are effective ways to improve the bioavailability of EETs and inhibit or even resolve neuroinflammation in AD. 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) is a potent sEH inhibitor that has been shown to be effective in preclinical animal models of a variety of chronic inflammatory diseases. This study aims to further explore whether TPPU can alleviate AD neuroinflammation.
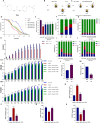
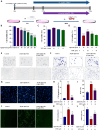
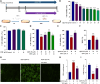
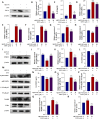
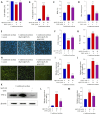
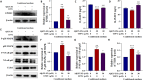
Methods: We established an Aβ42-transgenic Drosophila melanogaster model using the galactose-regulated upstream promoter element 4 (GAL4) / upstream active sequence (UAS) expression system and investigated the protective and anti-neuroinflammatory effects of TPPU against Aβ toxicity. We detected behavioral indexes (survival time, crawling ability, and olfactory memory) and biochemical indexes malondialdehyde (MDA) content and superoxide dismutase (SOD) activity in brain tissues of Aβ42 transgenic flies. Finally, we explored the anti-neuroinflammatory effect of TPPU and its possible mechanism by stimulating cocultures of human SH-SY5Y cells and HMC3 cells with Aβ(25-35) to model neuronal cell inflammation, and evaluated the cells by fluorescence microscopy, ELISA, Western Blot, and Real-time PCR.
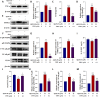
Results: We found that TPPU improved the survival time, crawling ability, and olfactory memory of Aβ42-transgenic flies. We also observed reduction of MDA content and elevation of SOD activity in the brain tissues of these flies. In human cell models, we found that TPPU improved cell viability, reduced cell apoptosis, decreased lipid oxidation, inhibited oxidative damage, thus playing a neuroprotective role. The inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-18 (IL-18) were downregulated, and the mRNA expression of the M2 microglia markers CD206 and SOCS3 were upregulated by TPPU; thus, TPPU inhibited neuroinflammatory responses. TPPU exerted neuroprotective and anti-inflammatory effects by decreasing the protein expression of the sEH-encoding gene EPHX2 and increasing the levels of 11,12-epoxyeicosatrienoic acid (11,12-EET) and 14,15-epoxyeicosatrienoic acid (14,15-EET). The inhibitory effect of TPPU on Aβ(25-35)-mediated neuroinflammation was associated with inhibition of the toll like receptor 4 (TLR4)/nuclear transcription factor-κB (NF-κB) pathway and p38 mitogen activated protein kinases (MAPK)/NF-κB pathway.
Conclusions: We report that the sEH inhibitor TPPU exerts neuroprotective and anti-neuroinflammatory effects in AD models, and it is expected that this drug could potentially be used for the prevention and treatment of AD.
Keywords: Drosophila melanogaster; Alzheimer’s disease; Cell; Neuroinflammation; Neuroprotection; TPPU.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: All listed authors consent to the submission. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer's Disease.Int J Mol Sci. 2025 Mar 8;26(6):2433. doi: 10.3390/ijms26062433. Int J Mol Sci. 2025. PMID: 40141075 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Buzhong Yiqi decoction improves inflammation and oxidative damage in autoimmune thyroiditis by inhibiting apoptosis via the SIRT1-Mediated Nrf2/NF-κB axis.J Ethnopharmacol. 2025 Jul 24;351:119967. doi: 10.1016/j.jep.2025.119967. Epub 2025 May 11. J Ethnopharmacol. 2025. PMID: 40360040
-
Inhibition of soluble epoxide hydrolase ameliorates cerebral blood flow autoregulation and cognition in alzheimer's disease and diabetes-related dementia rat models.Geroscience. 2025 Jun;47(3):4429-4449. doi: 10.1007/s11357-025-01550-8. Epub 2025 Feb 4. Geroscience. 2025. PMID: 39903369 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

