Extracellular vesicles: the "Trojan Horse" within breast cancer host microenvironments
- PMID: 40551109
- PMCID: PMC12183899
- DOI: 10.1186/s12943-025-02358-y
Extracellular vesicles: the "Trojan Horse" within breast cancer host microenvironments
Abstract
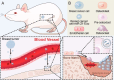
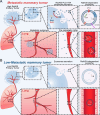
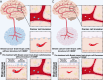
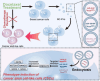
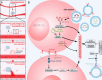
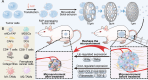
Breast cancer represents a significant global health concern among women. The intricate processes and pathways underlying metastasis contribute to the challenging prognoses experienced by some patients. Extracellular vesicles (EVs) are membrane-bound structures characterized by phospholipid bilayers, capable of secretion by a multitude of cell types. The contents of these vesicles encompass a diverse assortment of lipids, proteins, nucleic acids, and cellular metabolites. The tumor microenvironment (TME) comprises a complex network involving tumor cells, non-cancerous cells, and an array of molecules they generate and release. Components include the extracellular matrix, cancer-associated fibroblasts, inflammatory immune cells, tumor-associated vasculature, and EVs discharged by these cellular entities. Within the TME, EVs serve as a mechanism akin to the "Trojan Horse," exerting significant influence in tumor initiation, progression, metastasis, and responses to therapeutic interventions. EVs originating from tumor cells and associated entities within the TME bolster processes such as stimulating angiogenesis adjacent to tumor sites, establishing pre-metastatic niches in distant anatomical regions, and inducing transformative changes in cancer cells to acquire characteristics promoting invasion, angiogenesis, immune evasion, distant metastasis, and resistance to chemotherapy. Noteworthy is the unique capacity of EVs to traverse biological barriers due to their inherent biocompatibility, rendering them promising candidates for innovative drug delivery systems. This attribute presents an avenue to surmount the constraints of traditional cancer treatments. This scholarly inquiry delves into the pathogenic mechanisms of EVs in breast cancer and delves into prospective therapeutic interventions, offering a groundwork for forthcoming precision-guided therapies tailored to breast cancer.
Keywords: Breast cancer; Drug delivery vehicles; Drug resistance; Extracellular vesicles; Metastasis; Pre-metastatic niche; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: None. Consent for publication: None. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
"Small extracellular vesicles: messengers at the service of breast cancer agenda in the primary and distant microenvironments".J Exp Clin Cancer Res. 2025 Jul 21;44(1):216. doi: 10.1186/s13046-025-03471-y. J Exp Clin Cancer Res. 2025. PMID: 40691627 Free PMC article. Review.
-
Intercellular communication between extracellular vesicles from conditioned macrophages and breast cancer cells drives endocrine therapy resistance.Front Cell Dev Biol. 2025 Jun 3;13:1548724. doi: 10.3389/fcell.2025.1548724. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40567500 Free PMC article.
-
Biogenesis and functional implications of extracellular vesicles in cancer metastasis.Clin Transl Oncol. 2025 Jul;27(7):2913-2935. doi: 10.1007/s12094-024-03815-8. Epub 2024 Dec 20. Clin Transl Oncol. 2025. PMID: 39704958 Review.
-
Extracellular vesicles derived from Schwann cells to enhance bone and dental tissue regeneration: a literature review.J Nanobiotechnology. 2025 Jul 11;23(1):502. doi: 10.1186/s12951-025-03585-7. J Nanobiotechnology. 2025. PMID: 40646600 Free PMC article. Review.
-
Unveiling the Involvement of Extracellular Vesicles in Breast Cancer's Organotrophic Metastasis: Molecular Mechanisms and Translational Prospects.Int J Mol Sci. 2025 Jun 6;26(12):5430. doi: 10.3390/ijms26125430. Int J Mol Sci. 2025. PMID: 40564894 Free PMC article. Review.
References
-
- Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. - PubMed
-
- Miller KD, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

