Epigenetic modulation elicits an NK cell-mediated immune response in urothelial carcinoma
- PMID: 40551137
- PMCID: PMC12186328
- DOI: 10.1186/s10020-025-01264-9
Epigenetic modulation elicits an NK cell-mediated immune response in urothelial carcinoma
Abstract
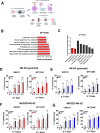
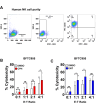
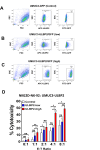
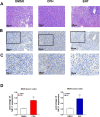
Urothelial carcinoma (UC), the second most prevalent cancer of the urothelial system, has demonstrated a well-developed ability to evade natural killer (NK) cell-mediated killing via various pathways, highlighting the need for innovative therapeutic approaches. Our study examines the role of cyproheptadine (CPH) and entinostat (ENT) as epigenetic modifiers in enhancing NK cell-mediated cytotoxicity against UC. Through our in vitro experiments, we observed that pre-treatment with CPH or ENT significantly increased NK cell-mediated elimination of UC cells, as demonstrated in NK cytotoxicity assay wherein UC cells were co-cultured with both NK-92 and human primary NK cells. This enhancement is attributed to the epigenetic activation of NKG2D ligands, notably through increased H3K27ac enrichment and reduced enrichment of H3K27me3 at the ULBP2 promoter region in UC cells. Additionally, ectopic expression of ULBP2 in UC cells further augmented their susceptibility to NK cell-mediated killing. Importantly, our syngeneic mouse model using MB49 tumor cells, showed that CPH or ENT treatments significantly reduced tumor growth via promoting NK cell infiltration into the tumor microenvironment. This increased infiltration is likely due to elevated levels of the NK-recruiting chemokine CCL3 in drug-treated UC cells. Collectively, these results underscore the potential of CPH and ENT to suppress tumor growth and enhance immune surveillance by modulating epigenetic pathways that boost NK cell activity, offering promising insights into new strategies for UC treatment.
Supplementary Information: The online version contains supplementary material available at 10.1186/s10020-025-01264-9.
Keywords: CCL3; CPH; Innate immunity; NK cells; NKG2DL; ULBP2; Urothelial carcinoma.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Review Board (IRB) of Taoyuan General Hospital (IRB no.: TYGH109026), Ministry of Health and Welfare and Institutional Animal Care and Use committee (IACUC) of the National Chung Cheng University, Taiwan. Consent for publication: All authors read the final manuscript and agreed to publish it. Competing interests: The authors declare no competing interests.
Figures



References
-
- Arai S, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32. - PubMed
-
- Armeanu S, et al. Natural killer cell-mediated Lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

