Elevated drain fluid calprotectin as a predictor of early anastomotic leakage in colorectal surgery
- PMID: 40551176
- PMCID: PMC12183818
- DOI: 10.1186/s12957-025-03899-8
Elevated drain fluid calprotectin as a predictor of early anastomotic leakage in colorectal surgery
Abstract
Background: Anastomotic leakage (AL) is a major cause of postoperative mortality following colorectal cancer surgery. This prospective investigation sought to establish the diagnostic utility of perioperative drain fluid calprotectin quantification in anticipating anastomotic complications following colorectal resections.
Methods: A consecutive cohort of 306 subjects undergoing anterior resection for sigmoid colon or rectal cancer were prospectively enrolled and stratified based on postoperative clinical outcomes: 25 cases developing AL (Group A) versus 281 without AL (Group B). Calprotectin levels in drainage fluid, serum C-reactive protein (CRP), and interleukin-6 (IL-6) were compared between the groups.
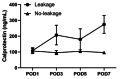
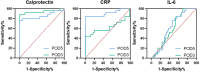
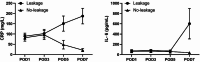
Results: The diagnosis of AL was made between the 3rd and 11th postoperative day (POD), with a mean diagnosis time of 7 days. Group A showed significantly higher calprotectin levels starting from POD3 (207 vs. 96 ng/mL, p < 0.0001). POD3 calprotectin concentrations exceeding 110 ng/mL demonstrated superior discriminative capacity, achieving 92% diagnostic sensitivity with 82% specificity for preclinical AL detection.
Conclusion: Early and persistent elevation of drain fluid calprotectin after colorectal surgery is a significant marker for AL, potentially offering an advantage over traditional inflammatory markers like CRP and IL-6 in the earlier prediction of AL. These findings provide valuable insights for improving postoperative management and patient outcomes.
Keywords: Anastomotic leakage; Calprotectin; Colorectal cancer; Drain fluid; Surgery.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study design was approved by the Second Affiliated Hospital of Zhejiang University School of Medicine (approval no: YS2020-055). Written informed consent was obtained from all individual participants included in the study. Consent for publication: All patients provided written informed consent before enrollment in the study and consent for publication. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

