Technologies for the point-of-care diagnosis of malaria: a scoping review
- PMID: 40551195
- PMCID: PMC12183878
- DOI: 10.1186/s40249-025-01329-1
Technologies for the point-of-care diagnosis of malaria: a scoping review
Abstract
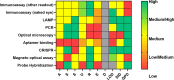
Background: Malaria continues to pose a significant health challenge, particularly in low-resource settings (LRS), where access to reliable and timely diagnostics is often limited. In this context, point-of-care (POC) in vitro diagnostics (IVDs) play a key role in supporting early detection and treatment. The aim of this scoping review was to better understand the landscape of malaria IVD technologies, with the aim of identifying both their strengths and limitations to guide and accelerate the development of POC diagnostics suitable for endemic regions and LRS. To support this analysis, the ASSURED (Affordability, Sensitivity, Specificity, User-friendliness, Rapidity, Equipment-free, Deliverability) criteria were applied to rank each technology in terms of its potential for POC applications in LRS.
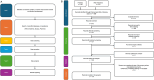
Methods: A literature search was conducted in PubMed and Web of Science for original research articles on malaria POC diagnostic devices published in English over the last 20 years (2003-2023). Records were screened based on eligibility criteria. For each paper, we identified biomarkers, biological specimens used, analytical methods, and readout technologies. Each record was ranked from low to high for its compatibility with the seven ASSURED criteria and for the Technology Readiness Level.
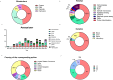
Results: The final dataset included 118 records. Of the methods considered, immunoassays were the most frequently reported (41.5%), followed by loop-mediated isothermal amplification (LAMP, 22.8%), polymerase chain reaction (PCR, 6.7%) and optical microscopy (4.2%). The limit of detection was highest for LAMP and PCR. Biomarkers employed for diagnosis included the Plasmodium parasite, parasite protein antigens and hemozoin. Blood was the most commonly employed biological specimen (76.2%), followed by urine and saliva (5.1%). Despite a focus on malaria IVDs for POC applications, only 8% of the records mentioned ASSURED criteria, with most studies manifesting low compatibility with the criteria.
Conclusions: Although meeting the ASSURED criteria remains challenging, microscopy is still the gold standard because of its diagnostic accuracy. Recent developments in low-cost, high-magnification lenses and innovative manufacturing techniques have enabled the production of microscopy devices in LRS. Combined with advancements in image processing and shape recognition through machine learning, there is strong potential for intellectual and economic investments to enhance microscopy for POC malaria diagnostics.
Keywords: ASSURED; Diagnosis; Malaria; Point-of-care; Scoping review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Figueroa-Miranda G, Feng L, Shiu SCC, Dirkzwager RM, Cheung YW, Tanner JA, Schöning MJ, et al. Aptamer-based electrochemical biosensor for highly sensitive and selective malaria detection with adjustable dynamic response range and reusability. Sens Actuators B Chem. 2018;255:235–43. 10.1016/j.snb.2017.07.117.
-
- Bronzan RN, Mcmorrow ML, Patrick Kachur S. Diagnosis of malaria challenges for clinicians in endemic and non-endemic regions. 2008. vol. 12 - PubMed
-
- World Health Organization. Global technical strategy for malaria 2016–2030. 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

