Α 6-month, multicenter, observational study investigating the treatment of venous thromboembolism in Greece (VICTORIA study)
- PMID: 40551211
- PMCID: PMC12183886
- DOI: 10.1186/s12959-025-00749-1
Α 6-month, multicenter, observational study investigating the treatment of venous thromboembolism in Greece (VICTORIA study)
Abstract
Background: Real-world data are needed to inform clinical practice with regards to anticoagulation treatment for persons with venous thromboembolism (VTE).
Objectives: To identify the type and duration of antithrombotic treatment in persons with VTE. Anticoagulation dosage and persistence/adherence were among the secondary objectives.
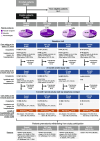
Methods: A multicenter, observational, prospective study conducted in Greek adults with VTE with two on-site visits -baseline and at three months- and a telephone follow-up at 6 months.
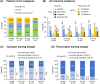
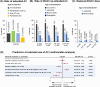
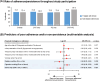
Results: A total of 600 eligible persons were enrolled. The index event was 'PE only' in 50%, 'DVT only' in 40%, and 'DVT+PE' in 10%. Risk factors were categorized as temporary major (21%), temporary minor (37%), and persistent (43%), with active cancer present in 18% of patients. All VTE patients received anticoagulants: 73% received oral anticoagulants (72% DOACs, 1% VKAs) and 70% received parenteral anticoagulants. Treatment was oral only in 30%, parenteral only in 27%, and both in 43%. The most common DOAC was apixaban (47%). Extended anticoagulation (>6 months) was administered to 41% with only 9% (18/198) of those on DOACs receiving a reduced dose. Persistent risk factors predicted extended anticoagulation, while diabetes, COVID-19, and temporary minor risk factors did not. Adherence/persistence rates were similar between DOAC and non-DOAC-treated patients.
Conclusion: VTE was mainly treated with a combination of parenteral and oral anticoagulants. DOACs, primarily apixaban, were the most common oral treatments. Forty percent of patients received extended anticoagulation, mostly at standard dosages. Adherence and persistence rates were high for both DOAC and non-DOAC treatments.
Keywords: Anticoagulant; Deep vein thrombosis; Duration of anticoagulation; Pulmonary embolism.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All participants signed an informed consent document. The final protocol, any amendments, and informed consent documentation were reviewed and approved by an Institutional Review Board for each site participating in the study. The study was conducted in accordance with legal and regulatory requirements and followed generally accepted research practices described in the Guidelines for Good Pharmacoepidemiology Practices issued by the International Society for Pharmacoepidemiology. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Supported by Pfizer Hellas SA. PS, IS, IA and DM are employees of Pfizer and may hold stock or stock options. EM, SK, GP, EF, GN, OK, GT, KF, CS, KI, VT, NK, IS, IK, GM, KN, DC, DS, CK, KM, IV, CK, PT and SFN declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. ED reports receiving support from Boehringer and MSD for attending meetings and travels. AG reports receiving consulting fees from Bayer, Servier, Medtronic and Gore. FZ reports participating as principal investigator at Alexandra General Hospital where payments were made to institute and receiving consulting fees, receiving honoraria for lectures and support for attending meetings and travel from AstraZeneca, Daiichi, Eli-Lilly, Merck, Pfizer, Genesis-Pharma, Novartis, MSD, Roche and Gilead. KK reports receiving grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Elpen, GlaxoSmithKline, Menarini and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Elpen, GlaxoSmithKline, Guidotti, Menarini, Pfizer and Sanofi, payment for lectures, presentations, manuscript writing from Alector Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Chiesi, Elpen, Gilead, GlaxoSmithKline, Guidotti, Menarini, Pfizer and Sanofi, participating on a safety data monitoring board or advisory board of Chiesi and being a board member of GOLD Assembly. DS being a board member of Hellenic Angiological Society. HM reports participating at clinical trials of Pfizer, receiving consulting fees from Sanofi and Novartis, honoraria for lectures from Amgen, AstraZeneca, Novartis, Pfizer and Sanofi, support for attending international congresses with travel, accommodation and meeting expenses from Novartis, Sanofi and Vianex and being a board member of Hellenic Society of Atherosclerosis and Hellenic Stroke Organization. TP receiving honoraria for lectures from Pfizer, support for attending international congresses with travel, accommodation and meeting expenses from Pfizer and being general secretary of Hellenic Phlebology Society. FM reports receiving support from Pfizer and Bayer for attending meetings and travels. Disclosure forms provided by the authors are available with the full text of this article at supplementary. No other potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer.Cochrane Database Syst Rev. 2018 Jun 19;6(6):CD006650. doi: 10.1002/14651858.CD006650.pub5. Cochrane Database Syst Rev. 2018. PMID: 29920657 Free PMC article.
-
Antithrombotic therapy for ambulatory patients with multiple myeloma receiving immunomodulatory agents.Cochrane Database Syst Rev. 2021 Sep 28;9(9):CD014739. doi: 10.1002/14651858.CD014739. Cochrane Database Syst Rev. 2021. PMID: 34582035 Free PMC article.
-
Pentasaccharides for the treatment of deep vein thrombosis.Cochrane Database Syst Rev. 2017 Dec 2;12(12):CD011782. doi: 10.1002/14651858.CD011782.pub2. Cochrane Database Syst Rev. 2017. PMID: 29199766 Free PMC article.
-
Oral anticoagulation in people with cancer who have no therapeutic or prophylactic indication for anticoagulation.Cochrane Database Syst Rev. 2017 Dec 29;12(12):CD006466. doi: 10.1002/14651858.CD006466.pub6. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Oct 8;10:CD006466. doi: 10.1002/14651858.CD006466.pub7. PMID: 29285754 Free PMC article. Updated.
-
Effect of testing for cancer on cancer- or venous thromboembolism (VTE)-related mortality and morbidity in people with unprovoked VTE.Cochrane Database Syst Rev. 2021 Oct 1;10(10):CD010837. doi: 10.1002/14651858.CD010837.pub5. Cochrane Database Syst Rev. 2021. PMID: 34597414 Free PMC article.
References
-
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–64. - PubMed
-
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019;54:1901647. - PubMed
-
- Mazzolai L, Aboyans V, Ageno W, Agnelli G, Alatri A, Bauersachs R, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2017;39:4208–18. - PubMed
-
- Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing G-J, et al. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:2247–59. - PubMed
LinkOut - more resources
Full Text Sources

