Brain tissue electrical conductivity as a promising biomarker for dementia assessment using MRI
- PMID: 40551292
- PMCID: PMC12185248
- DOI: 10.1002/alz.70270
Brain tissue electrical conductivity as a promising biomarker for dementia assessment using MRI
Abstract
Introduction: Dementia, particularly Alzheimer's disease, involves cognitive decline linked to amyloid beta (Aβ) and tau protein aggregation. Magnetic resonance imaging (MRI)-based brain tissue conductivity, which increases in dementia, may serve as a non-invasive biomarker for protein aggregation. We investigate the relationship between MRI-based brain electrical conductivity, protein aggregation, cognition, and gene expression.
Methods: Brain conductivity maps were reconstructed and correlated with PET protein signals, cognitive performance, and plasma protein levels. The diagnostic potential of conductivity for dementia was assessed, and transcriptomic analysis using the Allen Human Brain Atlas elucidated the underlying biological processes.
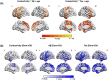
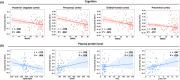
Results: Increased brain conductivity was associated with Aβ and tau aggregation in specific brain regions, cognitive decline, and plasma protein levels. Conductivity also improved dementia discrimination performance, and higher gene expression related to ion transport, cellular development, and signaling pathways was observed.
Discussion: Brain electrical conductivity shows promise as a biomarker for dementia, correlating with protein aggregation and relevant cellular processes.
Highlights: Brain tissue conductivity correlates with Aβ and tau aggregation in dementia. Brain tissue conductivity correlates with cognitive scores and GMV. CSF conductivity correlates with plasma protein levels. Combining conductivity with GMV improves dementia diagnosis accuracy. Gene expression in ion processes, cell development, and signaling links to conductivity.
Keywords: MRI; PET; dementia; electrical conductivity; positron emission tomography; protein aggregation.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting information.
Figures





Similar articles
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Impact of diabetes on the progression of Alzheimer's disease via trajectories of amyloid-tau-neurodegeneration (ATN) biomarkers.J Nutr Health Aging. 2025 Feb;29(2):100444. doi: 10.1016/j.jnha.2024.100444. Epub 2024 Dec 10. J Nutr Health Aging. 2025. PMID: 39662155 Free PMC article.
-
Two-Year Prognostic Utility of Plasma p217+tau across the Alzheimer's Continuum.J Prev Alzheimers Dis. 2023;10(4):828-836. doi: 10.14283/jpad.2023.83. J Prev Alzheimers Dis. 2023. PMID: 37874105
-
Cognitive variation reflects amyloid, tau, and neurodegenerative biomarkers in Alzheimer's disease.Geroscience. 2025 Jun;47(3):4763-4773. doi: 10.1007/s11357-025-01541-9. Epub 2025 Jan 28. Geroscience. 2025. PMID: 39873919 Free PMC article.
-
18F PET with florbetapir for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Nov 22;11(11):CD012216. doi: 10.1002/14651858.CD012216.pub2. Cochrane Database Syst Rev. 2017. PMID: 29164603 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

