Comparison of High-Dose versus Low-Dose Paclitaxel Drug-Coated Balloons for Native Femoropopliteal Artery Disease: An Analysis of the K-VIS ELLA Registry
- PMID: 40551590
- PMCID: PMC12206592
- DOI: 10.3349/ymj.2024.0166
Comparison of High-Dose versus Low-Dose Paclitaxel Drug-Coated Balloons for Native Femoropopliteal Artery Disease: An Analysis of the K-VIS ELLA Registry
Abstract
Purpose: Drug-coated balloons (DCBs) have demonstrated favorable outcomes in the treatment of femoropopliteal artery (FPA) disease. A variety of DCBs are currently available, with differing doses of antiproliferative agents and types of excipients. The objective of this study was to compare the efficacy and safety of high-dose versus low-dose paclitaxel DCBs for the treatment of FPA disease.
Materials and methods: We analyzed data from the multicenter the Korean Vascular Intervention Society Endovascular Therapy in Lower Limb Artery Diseases (K-VIS ELLA) registry, focusing on patients treated with a high-dose paclitaxel DCB (IN.PACT™) or low-dose paclitaxel DCB (Lutonix™ or Ranger™) for native vessel FPA disease. We used inverse probability of treatment weighting to adjust for confounding factors and conducted subgroup analyses based on lesion characteristics.
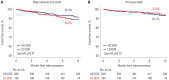
Results: Among 820 target limbs, 626 were treated with a high-dose paclitaxel DCB, and 194 were treated with a low-dose paclitaxel DCB. At 12 months, there were no significant differences in rates of freedom from clinically driven target lesion revascularization (TLR; 91.7% vs. 89.4%, log-rank p=0.35), major adverse limb event (MALE; 91.4% vs. 89.0%, log-rank p=0.31), or all-cause mortality (93.1% vs. 93.8%, log-rank p=0.79) between high-dose and low-dose groups. On multivariable analysis, the presence of chronic heart failure and chronic kidney disease were the only independent predictors of clinically driven TLR after DCB treatment.
Conclusion: In this multicenter cohort study of patients with complex FPA disease, there were no significant differences between high-dose DCB and low-dose DCB with respect to freedom from clinically driven TLR, MALE, or all-cause mortality at 12-month follow-up.
Keywords: Peripheral artery disease; balloon angioplasty; paclitaxel; popliteal artery.
© Copyright: Yonsei University College of Medicine 2025.
Figures

References
-
- Farhan S, Enzmann FK, Bjorkman P, Kamran H, Zhang Z, Sartori S, et al. Revascularization strategies for patients with femoropopliteal peripheral artery disease. J Am Coll Cardiol. 2023;81:358–370. - PubMed
-
- Scheinert D, Scheinert S, Sax J, Piorkowski C, Bräunlich S, Ulrich M, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45:312–315. - PubMed
-
- Tosaka A, Soga Y, Iida O, Ishihara T, Hirano K, Suzuki K, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59:16–23. - PubMed
-
- Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69:e71–e126. - PubMed
-
- Lee SJ, Lee HH, Ko YG, Ahn CM, Lee YJ, Kim JS, et al. Device effectiveness for femoropopliteal artery disease treatment: an analysis of K-VIS ELLA registry. JACC Cardiovasc Interv. 2023;16:1640–1650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

