Characterization of Apixaban Pharmacokinetics/Pharmacodynamics and Dose Assessment in Pediatric Patients with Congenital or Acquired Heart Disease
- PMID: 40551722
- PMCID: PMC12272319
- DOI: 10.1002/cpt.3689
Characterization of Apixaban Pharmacokinetics/Pharmacodynamics and Dose Assessment in Pediatric Patients with Congenital or Acquired Heart Disease
Abstract
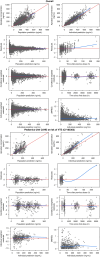
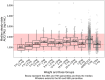
Apixaban is an oral direct inhibitor of factor Xa (FXa) that could be a treatment option for thromboembolism prevention in children with congenital or acquired heart disease (CAHD). Data from SAXOPHONE, a phase II pediatric study, were used to update a previously developed population pharmacokinetics (PPK) model and to assess the covariate effect of patient type on PK parameters while retaining previous covariates. Stochastic simulations were performed to assess whether the proposed dosing regimens in pediatric patients aged 28 days to < 18 years matched adult exposures. The relationship between anti-Factor Xa (AXA) and apixaban concentration, as well as apixaban concentration and chromogenic FX, were evaluated. Apixaban dose adjustment in response to the growth of pediatric patients and changes in age and weight were also assessed. Apixaban PK in pediatric patients with CAHD was well characterized by a 2-compartment model with first-order absorption, dose-dependent F1, and first-order elimination. Apixaban apparent clearance (CL/F) and apparent volume of distribution in the central compartment (Vc/F) increased with increasing body weight. Apixaban CL/F was lower in pediatric patients with CAHD compared to adults and other pediatric patients. The fixed dose by weight-tiered dosing regimen for pediatric patients with CAHD (28 days to < 18 years) achieved target exposures similar to adult VTE treatment and nonvalvular atrial fibrillation populations. A linear PK/PD relationship between apixaban and AXA was observed. Inhibition of FXa was observed across weight tiers. Apixaban dose adjustment in response to weight gain resulted in exposures similar to target adult exposures.
© 2025 Bristol Myers Squibb. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
A.A.‐H. is an employee of, received support for attending meetings/travel from, owns stock options in Bristol Myers Squibb, and has a leadership/fiduciary role with ASCPT. P.J., D.M., Z.W., W.C., B.H., B.M., and S.M. are employees of and hold stock in Bristol Myers Squibb. O.O. and E.L. are employees of Simulations Plus and were consultants to Pfizer and Bristol Myers Squibb in connection with the development of this manuscript. C.C. is an employee of Bristol Myers Squibb and served as Global Trial Manager (Operations) for the study (unpaid). R.M.P. has received research funding from the National Institutes of Health, the National Heart, Lung, and Blood Institute, Additional Ventures, and Friedreich's Ataxia Research Foundation; has received consultancy fees from Larimar Therapeutics, Inc.; has received travel support (paid to institution) for attending an American College of Cardiology meeting; has patents pending for Friedreich's ataxia and single‐ventricle heart disease biomarkers; and has served as a data safety monitoring board chair for a National Heart, Lung, and Blood Institute Friedreich's ataxia clinical trial. A.C.G. has received research funding from the National Institutes of Health and the National Heart, Lung, and Blood Institute. C.M. has received research funding from Bristol Myers Squibb and Pfizer; has received consultancy fees from Anthos, AstraZeneca, Bayer, Chiesi, Janssen, and Norgine; has received honoraria from Bayer; has participated in a data safety monitoring or advisory board for Bayer and Janssen; and has held a leadership or fiduciary role with the Pedi‐ATLAS Group. H.B. was an employee of and held stock in Bristol Myers Squibb. V.P. was an employee of and held stock in Bristol Myers Squibb, and is an employee of and holds stock in Bayer. All other authors declared no competing interests in this work. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, the United States Department of Health and Human Services, or Bristol Myers Squibb–Pfizer Alliance.
Figures


References
-
- Giglia, T.M. et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation 128, 2622–2703 (2013). - PubMed
-
- McCrindle, B.W. et al. Challenges and priorities for research: a report from the National Heart, Lung, and Blood Institute (NHLBI)/National Institutes of Health (NIH) working group on thrombosis in pediatric cardiology and congenital heart disease. Circulation 130, 1192–1203 (2014). - PubMed
-
- Bazzano, A.T. , Mangione‐Smith, R. , Schonlau, M. , Suttorp, M.J. & Brook, R.H. Off‐label prescribing to children in the United States outpatient setting. Acad. Pediatr. 9, 81–88 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

