A Prospective Study of Immune Response After COVID-19 or Vaccination and Correlation Between Avidity Index and Neutralizing Capacity
- PMID: 40551849
- PMCID: PMC12185213
- DOI: 10.1155/av/2265813
A Prospective Study of Immune Response After COVID-19 or Vaccination and Correlation Between Avidity Index and Neutralizing Capacity
Abstract
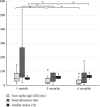
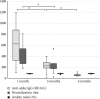
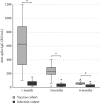
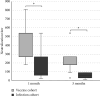
Background: Serological response is an important aspect of COVID-19, especially for evaluation of vaccine effect and risk of severe infection. The gold standard to assess this is analysis of neutralization capacity, but such assays are not widely available, with antibody levels often used as an approximation of the neutralizing ability. Avidity index is a measure of antibody function and avidity maturation contributes to long-lasting immunity. Objectives: To evaluate if the avidity index gives a better estimation of the neutralizing ability compared with anti-spike IgG levels and to compare the immune response between infection and vaccination against SARS-CoV-2. Study Design: Serum samples from prospectively included patients with either PCR-confirmed COVID-19 or COVID-19-naïve after vaccination were analyzed for anti-spike IgG, avidity index, and neutralizing titer at 1, 3, and 6 months after infection or vaccination. Results: A significant correlation between anti-spike IgG level and neutralization titer was found (Spearmanr s = 0.88, p < 0.001), but for the avidity index, the correlation was lower (Spearman r s = 0.62, p < 0.001). Anti-spike IgG level, avidity index, and neutralization titer were significantly higher in the vaccine cohort. Natural infection failed to yield high-avidity antibodies. Over time, the antibody levels and neutralization titer declined in both the vaccine and infection cohorts. Conclusion: Anti-spike IgG levels can be used as an estimation of the neutralizing titer, with the avidity index not contributing to a better estimation. There is a stronger initial immune response after vaccination, compared with natural infection. However, specific antibody levels decline over time, highlighting the importance of vaccine boosters.
Keywords: COVID-19; SARS-CoV-2; anti-spike IgG; avidity; immune response; neutralizing titer.
Copyright © 2025 Emma Löfström et al. Advances in Virology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- WHO. Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic 2023. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet... .
LinkOut - more resources
Full Text Sources
Miscellaneous

