Innate immunity, therapeutic targets and monoclonal antibodies in SARS-CoV-2 infection
- PMID: 40552037
- PMCID: PMC12184675
- DOI: 10.7717/peerj.19462
Innate immunity, therapeutic targets and monoclonal antibodies in SARS-CoV-2 infection
Abstract
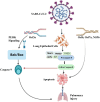
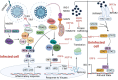
COVID-19 (coronavirus disease 2019), caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), stands as one of the most severe pandemics the world has ever faced in recent times. SARS-CoV-2 infection exhibits a wide range of symptoms, varying from severe manifestations to mild cases and even asymptomatic carriers. This diversity stems from a multitude of factors, including genetic predisposition, viral variants, and immune status. During SARS-CoV-2 infection, the immune system engages pattern recognition receptors, setting off a series of intricate signalling cascades. These cascades culminate in the activation of innate immune responses, including induction of type I and type III interferons. The emerging variants of SARS-CoV-2 pose challenges to the innate immune system defense. Therefore, investigating the innate immune response is crucial for effectively combating SARS-CoV-2 and its variants. The cyclic guanosine monophosphate-adenosine monophoshate synthase-stimulator of interferon genes (cGAS-STING) pathway, a critical innate immune mechanism, represents a promising target for intervention at multiple stages to reduce the severity and progression of SARS-CoV-2 infection. This review explores innate immunity in SARS-CoV-2 infection and other immune responses critical for SARS-CoV-2 defence. As part of the therapeutic approach, we extend our review to highlight monoclonal antibodies (mAbs) as emerging and effective therapeutics for controlling SARS-CoV-2 by targeting different stages of the innate immune system. A diverse range of mAbs has been explored to address specific targets within the innate immune pathways. A deep understanding of innate immunity and targeted monoclonal therapeutics will be instrumental in combating viruses and their variants, laying the foundation for enhanced treatment and therapeutic strategies.
Keywords: Innate immunity; Monoclonal antibody; SARS-CoV-2; STINGs; Signaling molecules.
©2025 Nazir et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
-
- Addetia A, Lieberman NAP, Phung Q, Hsiang TY, Xie H, Roychoudhury P, Shrestha L, Loprieno MA, Huang ML, Gale M, Jerome KR, Greninger AL. SARS-CoV-2 ORF6 disrupts bidirectional nucleocytoplasmic transport through interactions with Rae1 and Nup98. mBio. 2021;12(2):e00065-21. doi: 10.1128/mBio.00065-21. - DOI - PMC - PubMed
-
- Abers MS, Delmonte OM, Ricotta EE, Fintzi J, Fink DL, De Jesus AAA, Zarember KA, Alehashemi S, Oikonomou V, Desai JV, Canna SW, Shakoory B, Dobbs K, Imberti L, Sottini A, Quiros-Roldan E, Castelli F, Rossi C, Brugnoni D, Biondi A, Bettini LR, D’Angio M, Bonfanti P, Castagnoli R, Montagna D, Licari A, Marseglia GL, Gliniewicz EF, Shaw E, Kahle DE, Rastegar AT, Stack M, Myint-Hpu K, Levinson SL, Di Nubile MJ, Chertow DW, Burbelo PD, Cohen JI, Calvo KR, Tsang JS, NIAID COVID-19 Consortium. Su HC, Gallin JI, Kuhns DB, Goldbach-Mansky R, Lionakis MS, Notarangelo LD. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;6(1):e144455. doi: 10.1172/jci.insight.144455. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

