iMSC-Derived Extracellular Vesicles Improve Atopic Dermatitis by Augmenting Skin Barrier Integrity and Inhibiting Inflammation, Pruritus and Th2 Immune Responses
- PMID: 40552105
- PMCID: PMC12183344
- DOI: 10.1002/jex2.70067
iMSC-Derived Extracellular Vesicles Improve Atopic Dermatitis by Augmenting Skin Barrier Integrity and Inhibiting Inflammation, Pruritus and Th2 Immune Responses
Abstract
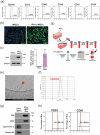
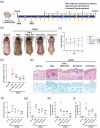
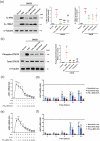
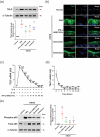
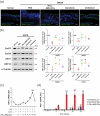
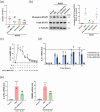
Atopic dermatitis (AD) is a chronic inflammatory disease characterized by severe itching and eczematous lesions. Despite various treatments, AD patients experience side effects and fail to achieve full remission. This study investigated the therapeutic potential of extracellular vesicles (EVs) derived from IFN-γ-primed induced mesenchymal stem cells (IFN-γ-iMSC-EVs) in a 2,4-dinitrochlorobenzene (DNCB)-induced AD mouse model. We also examined whether IFN-γ-iMSC-EVs could suppress IL-4/13-induced Th2 responses in keratinocytes. The therapeutic outcome of IFN-γ-iMSC-EVs was comparable to or more effective than baricitinib or clobetasol. While severe weight loss was observed in mice treated with clobetasol, no significant weight reduction occurred in those receiving IFN-γ-iMSC-EVs. Histological analysis demonstrated reduced skin thickness, decreased infiltration of mast cells and inflammatory cells, and suppression of the Th2 immune response, as evidenced by decreased signalling of IL-4, IL-13, and IL-31. IFN-γ-iMSC-EVs also led to a greater reduction in inflammation and pruritus compared to baricitinib and clobetasol. Additionally, skin barrier integrity and epidermal protein expression were improved in IFN-γ-iMSC-EVs. In IL-4/13-stimulated keratinocytes, the decrease in JAK1/2 gene expression and the increase in Keratin 1 gene expression were more prominent in IFN-γ-iMSC-EVs than in baricitinib. The results suggest that IFN-γ-iMSC-EVs have the potential to inhibit AD progression and represent a novel therapeutic option for AD.
Keywords: IFN‐γ; Th2 immune response; atopic dermatitis; extracellular vesicles; mesenchymal stem cells.
© 2025 The Author(s). Journal of Extracellular Biology published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
Soo Kim is the chief executive officer of Brexogen Inc. Other authors declare no conflicts of interest.
Figures

Similar articles
-
Extracellular vesicles from IFN-γ-primed mesenchymal stem cells repress atopic dermatitis in mice.J Nanobiotechnology. 2022 Dec 10;20(1):526. doi: 10.1186/s12951-022-01728-8. J Nanobiotechnology. 2022. PMID: 36496385 Free PMC article.
-
INDUCED PLURIPOTENT STEM CELL-DERIVED MESENCHYMAL STEM CELLS-DERIVED EXTRACELLULAR VESICLES ATTENUATE LPS-INDUCED LUNG INJURY AND ENDOTOXEMIA IN MICE.Shock. 2024 Aug 1;62(2):294-303. doi: 10.1097/SHK.0000000000002381. Epub 2024 May 23. Shock. 2024. PMID: 38813932 Free PMC article.
-
Extracellular Vesicles from miR-146a Overexpressing Mesenchymal Stem Cells Attenuate Imiquimod-Induced Psoriasis by Regulating Cytokine Expression.Iran J Immunol. 2025 Jun 23;22(2):119-130. doi: 10.22034/iji.2025.104576.2909. Online ahead of print. Iran J Immunol. 2025. PMID: 40546034
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Aghayan, A. H. , Mirazimi Y., Fateh K., Keshtkar A., Rafiee M., and Atashi A.. 2024. “Therapeutic Effects of Mesenchymal Stem Cell‐Derived Extracellular Vesicles in Sepsis: A Systematic Review and Meta‐Analysis of Preclinical Studies.” Stem Cell Reviews and Reports 20, no. 6: 1480–1500. 10.1007/s12015-024-10741-3. - DOI - PubMed
-
- Aviv, H. , Herzinger T., and Molin S.. 2020. “Skin Barrier Dysfunction in Contact Dermatitis and Atopic Dermatitis‐Treatment Implications.” Current Treatment Options in Allergy 7, no. 3: 390–402. 10.1007/s40521-020-00264-w. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
