Outcomes for high-risk defining events in follicular lymphoma following frontline immunochemotherapy
- PMID: 40552131
- PMCID: PMC12182845
- DOI: 10.1016/j.bneo.2024.100044
Outcomes for high-risk defining events in follicular lymphoma following frontline immunochemotherapy
Abstract
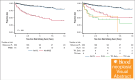
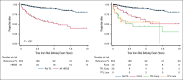
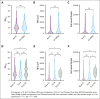
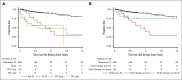
Progression of follicular lymphoma (FL) or transformation (TFL) within 24 months of immunochemotherapy (ICT) represent high-risk defining events (HRDE) with poor overall survival (OS). We examined baseline clinical characteristics, imaging, and outcomes for patients experiencing HRDE with newly diagnosed FL requiring ICT. HRDE groups were: relapse or progression of FL within 24 months (FL24), early TFL (transformation <24 months of ICT), late TFL (transformation >24 months of ICT).433 patients were categorized as reference FL (Ref FL), n = 352 (no HRDE); FL24, n = 43; early TFL, n = 29; late TFL, n = 9. Chemotherapy included bendamustine (63%), CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone) (27%), or CVP (cyclophosphamide, vincristine, prednisone) (10%); 85% received rituximab/15% obinutuzumab and 48% received maintenance therapy. Compared with Ref FL group, OS from HRDE was inferior for FL24 (hazard ratio [HR], 3.93; 95% confidence interval [CI], 2.14-7.23), early TFL (HR, 8.16; 95% CI, 4.38-15.2), and late TFL (HR, 8.23; 95% CI, 3.18-21.25). OS from HRDE was inferior for early TFL compared with FL24 (HR, 2.08; 95% CI, 1.02-4.21). In multivariable analysis, performance status, lactate dehydrogenase, beta-2-microglobulin and grade 3A were associated with early TFL. Clinical characteristics did not differentiate early TFL from FL24. Maximum standardized uptake value was higher in early TFL but not FL24 compared to Ref FL. Early TFL and FL24 represent different HRDEs and are associated with inferior OS. Distinguishing early TFL from FL24 is important for biomarker development, management and to develop and interpret trials in this area of unmet need.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.W.D.T. reports research funding from BeiGene, Cellectar, and Janssen. D.T. reports research funding from Janssen and Roche; and honoraria/speaker bureau fees from Roche, Janssen, Takeda, Amgen, CSL, EUSA, and Antengene. C.Y.C. reports honoraria/advisory/consulting fees from Roche, Janssen, Gilead, AstraZeneca, Lilly, BeiGene, Menarini, Dizal, AbbVie, Genmab, Sobi, and Bristol Myers Squibb; and research funding from Bristol Myers Squibb, Roche, AbbVie, MSD, and Lilly. E.H. reports research funding (paid to institution) from Roche, Bristol Myers Squibb, Merck KgA, AstraZeneca, TG Therapeutics, and Merck; consultancy or advisory role fees (paid to institution) from Roche, Merck Sharp & Dohme, AstraZeneca, Gilead, Antengene, Novartis, Regeneron, Janssen, Specialised Therapeutics, and Sobi; and travel expenses from AstraZeneca. The remaining authors declare no competing financial interests.
Figures
References
-
- Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. New Eng J Med. 2017;377(14):1331–1344. - PubMed
-
- Dinnessen MAW, Maas C, Tonino SH, et al. Causes of death of patients with follicular lymphoma in the Netherlands by stage and age groups: a population-based study in the pre- and post-rituximab era. Leukemia. 2022;36(5):1416–1420. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

