Progress in structure-based drug development targeting chemokine receptors
- PMID: 40552145
- PMCID: PMC12183272
- DOI: 10.3389/fphar.2025.1603950
Progress in structure-based drug development targeting chemokine receptors
Abstract
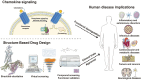
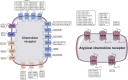
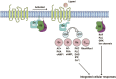
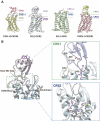
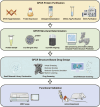
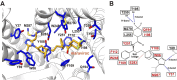
As a critical subfamily of G protein-coupled receptors (GPCRs), chemokine receptors (CCRs) play pivotal regulatory roles in immune cell migration, inflammatory modulation, tissue regeneration, and tumor microenvironment (TME) remodeling. By specifically recognizing chemokine ligands, CCRs orchestrate immune cell trafficking and tissue positioning, with functional dysregulation implicated in infectious diseases, autoimmune disorders, neurodegenerative pathologies, and cancer. These receptors thus represent promising therapeutic targets. Recent breakthroughs in cryo-electron microscopy (cryo-EM) and computational chemistry have enabled high-resolution structural analysis and dynamic conformational modeling of CCRs, establishing a robust foundation for structure-based drug design (SBDD). This review synthesizes current advances in CCR biology, structural mechanisms, disease involvement, and targeted drug development, providing theoretical insights and technical frameworks for future research.
Keywords: CCRs; GPCR; cryo-EM; drug discovery; structure-based drug design (SBDD).
Copyright © 2025 Wang, Qu, Xiao, Liu, Sun and Ping.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Agresti N., Lalezari J. P., Amodeo P. P., Mody K., Mosher S. F., Seethamraju H., et al. (2021). Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: case series of four critically ill patients treated with leronlimab. J. Transl. Autoimmun. 4, 100083. 10.1016/j.jtauto.2021.100083 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

