Molecular pharmacokinetic mechanism of quercetin-encapsulated polymeric micelles in alleviating cisplatin-induced nephrotoxicity and enhancing antineoplastic effects
- PMID: 40552152
- PMCID: PMC12183507
- DOI: 10.3389/fphar.2025.1590688
Molecular pharmacokinetic mechanism of quercetin-encapsulated polymeric micelles in alleviating cisplatin-induced nephrotoxicity and enhancing antineoplastic effects
Abstract
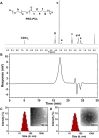
Introduction: Cisplatin (DDP), a platinum-based chemotherapy drug, shows broad antineoplastic activity, however, its clinical use is limited by dose-dependent nephrotoxicity, a major challenge in cancer therapy. The purpose of this study was to investigate the mechanism by which quercetin-polyethylene glycol-polycaprolactone (Que-PEG-PCL) micelles simultaneously enhance the cytotoxicity of DDP against cancer cells and reduce its nephrotoxicity.
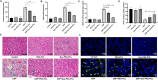
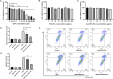
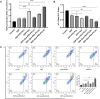
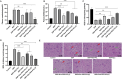
Methods: Rodent models and HEK293 cells were used to evaluate the renoprotective effects of Que-PEG-PCL micelles. Pharmacokinetics focused on OCT2-mediated renal DDP disposition. Antitumor activity was assessed in CT26 cells and syngeneic tumors. Key assessments included oxidative stress, apoptosis, renal markers, and histopathology.
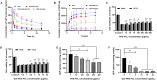
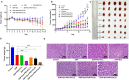
Results: Que-PEG-PCL reduced DDP-induced nephrotoxicity, lowering creatinine and BUN to 42% and 38%. It also reduced oxidative stress and improved antioxidant activity. DDP plasma exposure increased to 323%, with renal clearance reduced to 14%, due to OCT2 inhibition. In a CT26 syngeneic model, combination therapy inhibited tumor volume by 84% compared to control group.
Discussion: Que-PEG-PCL enhanced DDP's therapeutic window by limiting renal accumulation and promoting tumor cell apoptosis. This dual-action strategy provides a novel approach for improving the clinical efficacy of DDP-based cancer therapy.
Keywords: antitumor potency; cisplatin; nephrotoxicity; polymeric micelles; quercetin.
Copyright © 2025 Hao, Huo, Li, Wang, Wu, Song, Zhang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

