Efficacy and safety of immunotherapy or antiangiogenic agent-based treatment strategies versus chemotherapy as first-line treatment for extensive-stage small cell lung cancer: a network meta-analysis
- PMID: 40552156
- PMCID: PMC12183167
- DOI: 10.3389/fphar.2025.1539246
Efficacy and safety of immunotherapy or antiangiogenic agent-based treatment strategies versus chemotherapy as first-line treatment for extensive-stage small cell lung cancer: a network meta-analysis
Abstract
Objective: Immune checkpoint inhibitors (ICIs) combined with etoposide-platinum are recommended as the standard first-line therapy for extensive-stage small cell lung cancer (ES-SCLC). Despite the potential of antiangiogenic agents to enhance treatment efficacy, the optimal combination pattern remains unclear. This meta-analysis explores existing treatment strategies involving ICIs or antiangiogenic agents in ES-SCLC.
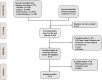
Methods: Hazard ratios (HRs) and odds ratios (ORs) were generated by R software. The outcomes of overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse events of grade 3 or higher (Grade ≥3 AEs) were analyzed. The included trials were classified in terms of different treatment strategies, including ICI + Chemotherapy (ICI + Chemo), ICI + ICI + Chemotherapy (ICI + ICI + Chemo), ICI + Antiangiogenic agent + Chemotherapy (ICI + Antiangio + Chemo), Antiangiogenic agent + Chemotherapy (Antiangio + Chemo), and Chemotherapy (Chemo).
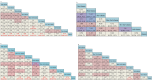
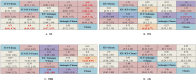
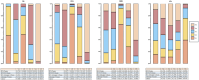
Results: A total of 13 randomized controlled trials (RCTs) involving 6,822 patients were included in the analysis. The drug combination patterns included ipilimumab, durvalumab, adebrelimab, atezolizumab, socazolimab, pembrolizumab, serplulimab, tislelizumab, toripalimab, durvalumab + tremelimumab, tiragolumab + atezolizumab, benmelstobart + anlotinib, bevacizumab + atezolizumab, anlotinib, bevacizumab in combination with chemotherapy. The antiangiogenic agent-containing regimen benmelstobart + anlotinib + chemotherapy demonstrated the highest potential to achieve superior PFS and OS versus chemotherapy. The group meta-analysis also showed that ICI + Chemo, ICI + ICI + Chemo, and ICI + Antiangio + Chemo presented significantly better OS. Additionally, ICI + Antiangio + Chemo achieved better PFS with the lowest HR of 0.37 and the best ORR of 2.08 versus chemotherapy. Patients treated with benmelstobart + anlotinib + chemotherapy, durvalumab + tremelimumab + chemotherapy, and anlotinib + chemotherapy experienced a higher likelihood of grade ≥3 AEs.
Conclusion: For individuals with ES-SCLC, ICI + Antiangio + Chemo was identified as an optimal treatment option due to better OS, PFS, and ORR. Benmelstobart + anlotinib + chemotherapy demonstrated a better survival benefit than chemotherapy. The toxicity of ICI + Antiangio + Chemo was acceptable but needed careful attention. These findings clarified the roles of ICIs and antiangiogenic agent-based treatment strategies in this population.
Keywords: antiangiogenesis; chemotherapy; immunotherapy; network meta-analysis; small cell lung cancer.
Copyright © 2025 Wang, Yang, Zhao, Zhang, Xuan and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict.
Figures




References
-
- Cheng Y., Fan Y., Zhao Y., Huang D., Li X., Zhang P., et al. (2024b). Tislelizumab plus platinum and etoposide versus placebo plus platinum and etoposide as first-line treatment for extensive-stage SCLC (RATIONALE-312): a multicenter, double-blind, placebo-controlled, randomized, phase 3 clinical trial. J. Thorac. Oncol. 19, 1073–1085. 10.1016/j.jtho.2024.03.008 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

