Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024
- PMID: 40552159
- PMCID: PMC12184384
- DOI: 10.3389/fphar.2025.1516449
Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024
Abstract
Introduction: Irinotecan is a widely used chemotherapeutic agent for treating colorectal, pancreatic, and ovarian cancers. Despite its therapeutic efficacy, the safety profile of irinotecan necessitates continuous pharmacovigilance due to its association with severe adverse drug events (ADEs). Given its global use, cross-national signal detection may reveal region-specific risks or unrecognized adverse effects.
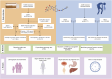
Methods: We conducted a retrospective pharmacovigilance analysis of irinotecan-associated ADEs using two large spontaneous reporting systems: the U.S. FDA Adverse Event Reporting System (FAERS) and the Japan Adverse Drug Event Report (JADER) database. ADE reports between 2004 and 2024 were extracted. Disproportionality analyses were performed using four methods: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and Multi-item gamma Poisson shrinker (MGPS).
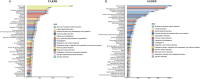
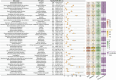
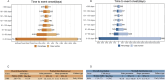
Results: A total of 11,344 ADE reports from FAERS and 7,822 from JADER were identified. These reports involved 27 system organ classes (SOCs). In FAERS, the most frequently affected SOC was gastrointestinal disorders (n = 6,888), while in JADER it was blood and lymphatic system disorders (n = 3,389). Disproportionality analysis revealed 388 and 67 preferred terms (PTs) significantly associated with irinotecan in FAERS and JADER, respectively, with 38 overlapping signals. These included both expected ADEs (e.g., neutropenia, diarrhea, thrombocytopenia, stomatitis) and unexpected signals such as second primary malignancies, hyperammonaemia, and hiccups. Notable FAERS-specific signals included skin toxicity (n=100, ROR 33.89 (27.79-41.34), PRR 33.80, EBGM05 28.03, IC025 4.76), aphasia [n=65, ROR 3.57 (2.8-4.55), PRR 3.56, EBGM05 2.90, IC025 1.47], and hepatic failure [n=56, ROR 3.09 (2.38-4.02), PRR 3.09, EBGM05 2.48, IC025 1.24], while JADER-specific signals included fatigue [n=73, ROR 4.69 (3.71-5.93), PRR 4.67, EBGM05 3.57, IC025 0.51], hyperammonaemia [n=67, ROR 7.24 (5.56-9.27), PRR 7.21, EBGM05 5.32, IC025 1.10], and cholinergic syndrome [n=27, ROR 5.54 (3.76-8.16), PRR 5.53, EBGM05 3.61, IC025 0.74]. Over half of all reported ADEs occurred within one month of irinotecan administration (53.1% in FAERS, 61.7% in JADER). The median time to onset was 28 days [IQR 9-76] in FAERS and 17 days [IQR 9-57] in JADER.
Discussion: This comparative analysis revealed multiple consistent and unexpected signals related to irinotecan use. The findings emphasize the importance of region-specific pharmacovigilance and the need for heightened awareness of both labeled and unlabeled toxicities. Our results support continued monitoring and further investigation into temporal patterns and regional differences in irinotecan-related adverse events to enhance clinical safety.
Keywords: FAERS; JADER; adverse drug event; irinotecan; real-world pharmacovigilance analysis.
Copyright © 2025 Lou, Chen, Cui, Zhang, Zhu, Zhou, Ou and Zou.
Conflict of interest statement
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adjei A. A., Klein C. E., Kastrissios H., Goldberg R. M., Alberts S. R., Pitot H. C., et al. (2000). Phase I and pharmacokinetic study of irinotecan and docetaxel in patients with advanced solid tumors: preliminary evidence of clinical activity. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 18 (5), 1116–1123. 10.1200/JCO.2000.18.5.1116 - DOI - PubMed
-
- Ando M., Eguchi K., Shinkai T., Tamura T., Ohe Y., Yamamoto N., et al. (1997). Phase I study of sequentially administered topoisomerase I inhibitor (irinotecan) and topoisomerase II inhibitor (etoposide) for metastatic non-small-cell lung cancer. Br. J. Cancer 76 (11), 1494–1499. 10.1038/bjc.1997.584 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

