Revealing the mechanisms of warfarin-induced vascular calcification through metabolomics and network toxicology
- PMID: 40552162
- PMCID: PMC12183513
- DOI: 10.3389/fphar.2025.1554987
Revealing the mechanisms of warfarin-induced vascular calcification through metabolomics and network toxicology
Abstract
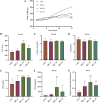
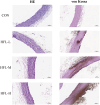
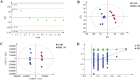
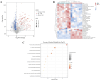
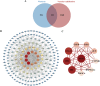
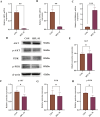
Warfarin is widely used in clinical anticoagulation therapy, but the exact mechanism by which it induces vascular calcification (VC) remains unclear. This study aimed to explore the mechanisms of warfarin-induced VC using metabolomics and network toxicology approaches. Initially, normal rats were orally administered warfarin for 2 weeks, and VC was then assessed by serum biochemistry and histopathology. Subsequently, non-targeted metabolomics was performed to analyze serum metabolite changes. Finally, network toxicology analysis was conducted to identify key targets and signaling pathways associated with warfarin-induced VC, which were further validated using molecular docking, qRT-PCR, and western blot analyses. The results indicated that warfarin induced aortic calcification in rats, and metabolomics identified 32 differential metabolites, mainly involved in pathways such as primary bile acid biosynthesis, steroid hormone biosynthesis, and amino acid metabolism. Network toxicology analysis, molecular docking, and experimental validation showed that warfarin may induce VC by modulating the targets AKT1, TP53, and HSP90AA1, thereby influencing the PI3K-AKT signaling pathway. This study reveals the potential molecular mechanisms underlying warfarin-induced VC, laying a foundation for further mechanistic investigations and providing important insights for the rational clinical application of warfarin.
Keywords: metabolomics; molecular mechanisms; network toxicology; vascular calcification; warfarin.
Copyright © 2025 Zhang, Jia, Feng, Xu, Zhang, Bai, Chen and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Avula V., Mok Y., Ejiri K., Van't Hof J., Whelton S. P., Hoogeveen R. C., et al. (2025). Inflammatory markers and calcification of coronary arteries, aorta and cardiac valves: findings from the atherosclerosis risk in communities study. Am. J. Prev. Cardiol. 21, 100946. 10.1016/j.ajpc.2025.100946 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

