TREM2 signaling pathway in sepsis-induced acute lung injury: physiology, pathology, and therapeutic applications
- PMID: 40552184
- PMCID: PMC12183286
- DOI: 10.3389/fmed.2025.1546292
TREM2 signaling pathway in sepsis-induced acute lung injury: physiology, pathology, and therapeutic applications
Abstract
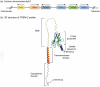
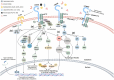
Sepsis emerges as a formidable and life-threatening condition, born from an unregulated immune response to infection, presenting a significant challenge to global health. A notable complication of sepsis is acute lung injury (ALI), marked by profound hypoxia, rampant inflammation, and the accumulation of fluid within the pulmonary system. ALI harbors the potential to escalate into acute respiratory distress syndrome (ARDS), thereby exacerbating the severity of sepsis. The triggering receptor expressed on myeloid cells 2 (TREM2), predominantly situated within various myeloid cell types, plays a pivotal role in the modulation of neurodegeneration, inflammation, neoplasms, and other pathologies. Recent investigations have illuminated TREM2's considerable involvement in septic lung injury; however, the precise mechanisms and therapeutic implications within this context demand further scrutiny. This article endeavors to elucidate the intricate interplay between sepsis, lung injury, and TREM2's role in immune modulation. It will furnish an overview of the TREM2 signaling pathway's functions and mechanisms in both physiological and septic lung injury scenarios, while also evaluating the current status and advancements in TREM2-targeted therapies.
Keywords: ALI; TREM2; immunomodulation; sepsis; therapeutic application.
Copyright © 2025 Shen, He and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pleiotropic role of TLR2-mediated signaling in the protection of psoralidin against sepsis-induced acute lung injury.Phytomedicine. 2025 Aug;144:156443. doi: 10.1016/j.phymed.2025.156443. Epub 2025 Feb 1. Phytomedicine. 2025. PMID: 40516289
-
Positioning for acute respiratory distress in hospitalised infants and children.Cochrane Database Syst Rev. 2022 Jun 6;6(6):CD003645. doi: 10.1002/14651858.CD003645.pub4. Cochrane Database Syst Rev. 2022. PMID: 35661343 Free PMC article.
-
Crosstalk between lung and extrapulmonary organs in sepsis-related acute lung injury/acute respiratory distress syndrome.Ann Intensive Care. 2025 Jul 14;15(1):97. doi: 10.1186/s13613-025-01513-4. Ann Intensive Care. 2025. PMID: 40658295 Free PMC article. Review.
-
Xuebijing Injection Alleviates Sepsis-Induced Acute Lung Injury by Inhibition of Cell Apoptosis and Inflammation Through the Hippo Pathway.J Inflamm Res. 2025 Jun 12;18:7717-7733. doi: 10.2147/JIR.S523991. eCollection 2025. J Inflamm Res. 2025. PMID: 40529898 Free PMC article.
-
Macrophage TRIM21 knockout inhibits septic acute lung injury by downregulating autophagy regulator protein ubiquitination.Autophagy. 2025 Jun 24:1-20. doi: 10.1080/15548627.2025.2519063. Online ahead of print. Autophagy. 2025. PMID: 40509575
References
-
- Abe T, Yamakawa K, Ogura H, Kushimoto S, Saitoh D, Fujishima S, et al. Epidemiology of sepsis and septic shock in intensive care units between sepsis-2 and sepsis-3 populations: sepsis prognostication in intensive care unit and emergency room (SPICE-ICU). J Inten Care. (2020) 8:44. 10.1186/s40560-020-00465-0 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

