RPL35A Downregulation Suppresses Hepatocellular Carcinoma Cell Proliferation via NCAPG2 Inactivation
- PMID: 40552444
- PMCID: PMC12185999
- DOI: 10.1002/cam4.70985
RPL35A Downregulation Suppresses Hepatocellular Carcinoma Cell Proliferation via NCAPG2 Inactivation
Abstract
Background: Hepatocellular carcinoma (HCC) is a highly aggressive cancer with a poor prognosis. The molecular mechanisms underlying HCC progression remain poorly understood, prompting the need for novel therapeutic targets. RPL35A, a component of the 60S large ribosomal subunit, is a ribosomal protein involved in ribosome biogenesis and protein synthesis. Beyond its canonical role, increasing evidence suggests that ribosomal proteins such as RPL35A may also exert extraribosomal functions that contribute to tumorigenesis.
Methods: We investigated RPL35A expression in HCC using tissue samples and cell lines. RPL35A levels were correlated with clinicopathological features and prognosis in HCC patients. In vitro, we manipulated RPL35A expression in HCC cells using shRNA lentiviral vectors and assessed its effects on cell proliferation, migration and apoptosis. In vivo, we evaluated tumor growth using xenograft models. Gene expression analysis was conducted to identify downstream targets of RPL35A.
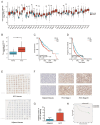
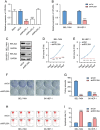
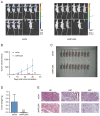
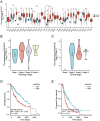
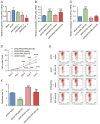
Results: RPL35A was significantly overexpressed in HCC tissues compared to normal liver, correlating with advanced disease stages and poorer prognosis. Knockdown of RPL35A in HCC cells inhibited cell proliferation, migration and invasion, while promoting apoptosis. In vivo, RPL35A silencing reduced tumor growth and size. Gene expression analysis identified NCAPG2 as a key downstream target of RPL35A. NCAPG2 expression was upregulated in HCC, and its knockdown reversed the oncogenic effects of RPL35A. Moreover, RPL35A overexpression increased NCAPG2 levels, promoting tumor progression. These findings suggest that the RPL35A/NCAPG2 axis is crucial in HCC development.
Conclusions: High expression of RPL35A is linked to poor prognosis in hepatocellular carcinoma. The regulation of NCAPG2 by RPL35A may represent a critical mechanism underlying RPL35A-driven tumor progression. Targeting the RPL35A/NCAPG2 pathway may offer a promising therapeutic strategy for HCC treatment.
Keywords: HCC; cell cycle arrest; molecular interplay; therapeutic target; tumor modulation.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Su G. L., Altayar O., O'Shea R., et al., “AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma,” Gastroenterology 162, no. 3 (2022): 920–934. - PubMed
-
- Sapisochin G. and Bruix J., “Liver Transplantation for Hepatocellular Carcinoma: Outcomes and Novel Surgical Approaches,” Nature Reviews Gastroenterology & Hepatology 14, no. 4 (2017): 203–217. - PubMed
-
- Maki H. and Hasegawa K., “Advances in the Surgical Treatment of Liver Cancer,” Bioscience Trends 16, no. 3 (2022): 178–188. - PubMed
-
- Sung H., Ferlay J., Siegel R. L., et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 71, no. 3 (2021): 209–249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

