Development and validation of highly sensitive ligand binding assay to measure soluble DLL3 concentration in human serum
- PMID: 40552557
- PMCID: PMC12203847
- DOI: 10.1080/17576180.2025.2518047
Development and validation of highly sensitive ligand binding assay to measure soluble DLL3 concentration in human serum
Abstract
Background: Delta-like protein 3 (DLL3) is considered to inhibit the Notch pathway in the tumorigenesis of small cell lung cancer (SCLC) and other neuroendocrine carcinomas, making it a potential therapeutic target in the treatment of cancer. Since the soluble form (sDLL3) is expected to be useful for predicting the status of DLL3 expression on tumors, analytical methods to measure sDLL3 are required.
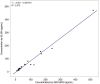
Research design and methods: Assay methods using ELISA and the SMCxPRO platform were developed to analyze sDLL3 concentration in human serum. The performance of the ELISA was evaluated and the SMCxPRO assay was fully validated, and the comparability of the 2 assays was assessed.
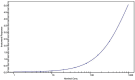
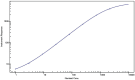
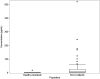
Results: The performance of the ELISA was acceptable, and in the SMCxPRO assay validation, all pre-defined validation acceptance criteria were met. The 2 assays were comparable within the range of quantification. Concentrations ranged from below the limit of quantification (<1.00 pg/mL) to 18.0 pg/mL for healthy volunteers and from 1.27 pg/mL to 519 pg/mL for SCLC patients by SMCxPRO assay.
Conclusions: Two sensitive assay methods to measure sDLL3 in human serum were successfully established. These assays have potential as novel blood-based assays to assess the status of DLL3 expression on tumors in humans.
Keywords: DLL3; Delta-like protein 3; ELISA; LBA; SMCxPRO; highly sensitive; lung cancer; validation.
Conflict of interest statement
Masanobu Nishidate: employee of Chugai Pharmaceutical Co., Ltd.
Chisato Yanagisawa: employee of Chugai Research Institute for Medical Science, Inc., the subsidiary of Chugai Pharmaceutical Co., Ltd.
Hiroki Irie: employee of Chugai Pharmaceutical Co., Ltd.
Kayo Aida: employee of Chugai Research Institute for Medical Science, Inc., the subsidiary of Chugai Pharmaceutical Co., Ltd.
Takashi Miyayama: employee of Chugai Pharmaceutical Co., Ltd.
Kimio Terao: employee of Chugai Pharmaceutical Co., Ltd.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No external writing assistance was utilized in the production of this manuscript.
Figures

Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Blood biomarkers for the non-invasive diagnosis of endometriosis.Cochrane Database Syst Rev. 2016 May 1;2016(5):CD012179. doi: 10.1002/14651858.CD012179. Cochrane Database Syst Rev. 2016. PMID: 27132058 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
