Neural dynamics of visual working memory representation during sensory distraction
- PMID: 40552615
- PMCID: PMC12187136
- DOI: 10.7554/eLife.99290
Neural dynamics of visual working memory representation during sensory distraction
Abstract
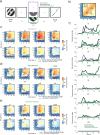
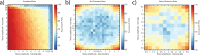
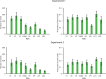
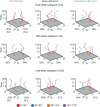
Recent studies have provided evidence for the concurrent encoding of sensory percepts and visual working memory (VWM) contents across visual areas; however, it has remained unclear how these two types of representations are concurrently present. Here, we reanalyzed an open-access fMRI dataset where participants memorized a sensory stimulus while simultaneously being presented with sensory distractors. First, we found that the VWM code in several visual regions did not fully generalize between different time points, suggesting a dynamic code. A more detailed analysis revealed that this was due to shifts in coding spaces across time. Second, we collapsed neural signals across time to assess the degree of interference between VWM contents and sensory distractors, specifically by testing the alignment of their encoding spaces. We find that VWM and feature-matching sensory distractors are encoded in coding spaces that do not fully overlap, but the separation decreases when distractors negatively impact behavioral performance in recalling the target. Together, these results indicate a role of dynamic coding and temporally stable coding spaces in helping multiplex perception and VWM within visual areas.
Keywords: dynamic coding; human; neural subspaces; neuroscience; sensory distraction; working memory.
© 2024, Degutis et al.
Conflict of interest statement
JD, SW, JS, JH No competing interests declared
Figures




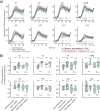


Update of
- doi: 10.1101/2024.04.12.589170
- doi: 10.7554/eLife.99290.1
- doi: 10.7554/eLife.99290.2
- doi: 10.7554/eLife.99290.3
Similar articles
-
Transformed Visual Working Memory Representations in Human Occipitotemporal and Posterior Parietal Cortices.eNeuro. 2025 Jul 9;12(7):ENEURO.0162-25.2025. doi: 10.1523/ENEURO.0162-25.2025. Print 2025 Jul. eNeuro. 2025. PMID: 40473470 Free PMC article.
-
Priority states modulate attentional interference from visual working memory representations: Electrophysiological evidence.Biol Psychol. 2025 Jul;199:109080. doi: 10.1016/j.biopsycho.2025.109080. Epub 2025 Jul 4. Biol Psychol. 2025. PMID: 40617340
-
Reappearing sensory input guides visual working memory prioritization.Atten Percept Psychophys. 2025 Oct;87(7):2129-2145. doi: 10.3758/s13414-025-03103-9. Epub 2025 Jun 11. Atten Percept Psychophys. 2025. PMID: 40500558 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Manipulating attentional priority creates a trade-off between memory and sensory representations in human visual cortex.bioRxiv [Preprint]. 2024 Sep 16:2024.09.16.613302. doi: 10.1101/2024.09.16.613302. bioRxiv. 2024. PMID: 39345376 Free PMC article. Preprint.
References
-
- Björck Ȧke, Golub GH. Numerical methods for computing angles between linear subspaces. Mathematics of Computation. 1973;27:579–594. doi: 10.1090/S0025-5718-1973-0348991-3. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

