Associations of semaglutide with Alzheimer's disease-related dementias in patients with type 2 diabetes: A real-world target trial emulation study
- PMID: 40552638
- PMCID: PMC12262134
- DOI: 10.1177/13872877251351329
Associations of semaglutide with Alzheimer's disease-related dementias in patients with type 2 diabetes: A real-world target trial emulation study
Abstract
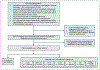
BackgroundAlmost half of the dementia cases are preventable. Semaglutide treats several medical conditions that are risk factors for dementia.ObjectiveWe aim to investigate if semaglutide is associated with a decreased risk of dementia.MethodsWe conducted emulation target trials based on a nationwide population-based database of patient electronic health records (EHRs) in the US among 1,710,995 eligible patients with type 2 diabetes (T2D) comparing semaglutide with other antidiabetic medications. First-time diagnosis of Alzheimer's disease-related dementia (ADRD) including vascular dementia, frontotemporal dementia, Lewy body dementia and other dementias were examined using Cox proportional hazards and Kaplan-Meier survival analyses during a 3-year follow-up. Models were adjusted by propensity-score matching.ResultsWe show that semaglutide was associated with a significantly reduced risk of overall ADRD incidence with a hazard ratio ranging from 0.54 (0.49-0.59) compared with insulin, 0.67 (0.61-0.74) compared with metformin, to 0.80 (0.72-0.89) compared with older generation glucagon-like peptide-1 agonists (GLP-1RAs). The association varied for specific dementia types, with significantly reduced risk of vascular dementia and no evidence of associations with frontotemporal and Lewy body dementias.ConclusionsThese findings provide evidence supporting protective effects of semaglutide on dementias in patients with T2D. Future works are needed to establish the causal relationships through randomized clinical trials and to characterize the underlying mechanisms.
Keywords: Alzheimer's disease related dementias; Alzheimer’s disease; Lewy body dementia; dementia; frontotemporal dementia; semaglutide; target trial emulation; type 2 diabetes; vascular dementia.
Conflict of interest statement
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: George Perry is an Editor-in-Chief of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review. The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
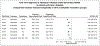


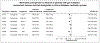
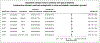
Similar articles
-
Associations of semaglutide with first-time diagnosis of Alzheimer's disease in patients with type 2 diabetes: Target trial emulation using nationwide real-world data in the US.Alzheimers Dement. 2024 Dec;20(12):8661-8672. doi: 10.1002/alz.14313. Epub 2024 Oct 24. Alzheimers Dement. 2024. PMID: 39445596 Free PMC article.
-
Glucagon-Like Peptide 1 Receptor Agonists and 13 Obesity-Associated Cancers in Patients With Type 2 Diabetes.JAMA Netw Open. 2024 Jul 1;7(7):e2421305. doi: 10.1001/jamanetworkopen.2024.21305. JAMA Netw Open. 2024. PMID: 38967919 Free PMC article.
-
Association of semaglutide with reduced incidence and relapse of cannabis use disorder in real-world populations: a retrospective cohort study.Mol Psychiatry. 2024 Aug;29(8):2587-2598. doi: 10.1038/s41380-024-02498-5. Epub 2024 Mar 14. Mol Psychiatry. 2024. PMID: 38486046 Free PMC article.
-
Selegiline for Alzheimer's disease.Cochrane Database Syst Rev. 2003;(1):CD000442. doi: 10.1002/14651858.CD000442. Cochrane Database Syst Rev. 2003. PMID: 12535396
-
Mini-Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2015 Mar 5;2015(3):CD010783. doi: 10.1002/14651858.CD010783.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 Jul 27;7:CD010783. doi: 10.1002/14651858.CD010783.pub3. PMID: 25740785 Free PMC article. Updated.
Cited by
-
Pathological mechanisms and treatment progression of Alzheimer's disease.Eur J Med Res. 2025 Jul 14;30(1):625. doi: 10.1186/s40001-025-02886-9. Eur J Med Res. 2025. PMID: 40660381 Free PMC article. Review.
References
-
- Murphy S, Kochanek K, Xu J, et al. Mortality in the United States, 2016. NCHS Data Brief 2017; 293: 1–8. - PubMed
-
- Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024; 404: 572–628. - PubMed
-
- Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch Neurol 2004; 61: 668–672. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

