Selective Iridium-Catalyzed Reductive Amination Inside Living Cells
- PMID: 40552662
- PMCID: PMC12374733
- DOI: 10.1021/jacs.5c08536
Selective Iridium-Catalyzed Reductive Amination Inside Living Cells
Abstract
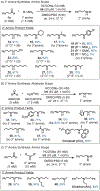
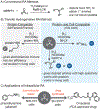
Given that amino groups are ubiquitous in bioactive molecules, abiotic routes to incorporate them into cellular species offer new opportunities to study and manipulate living systems. In the present work, we report the first biocompatible method to prepare 1°, 2°, or 3° amines selectively starting from an aldehyde and nitrogen precursor through iridium-catalyzed reductive amination. To prevent overalkylation, we developed a nontoxic self-immolative agent comprising 4-(1-aminoethyl)phenol that can condense with carbonyl groups and undergo 1,6-elimination upon reduction to the desired 1° amines. The use of an electron-poor half-sandwich Ir catalyst favored the formation of amine over alcohol products. To synthesize 2° or 3° amines, the aldehydes were combined with the appropriate 1° or 2° amine, respectively, under our standard reaction conditions. Our method is sufficiently mild to perform on proteins, as demonstrated by the conversion of aldehyde-containing allysine residues in bovine serum albumin to lysine. Importantly, we showed that Ir-catalyzed reductive amination could be applied inside living cells, such as by generating the alkaloid phenethylamine or calcium-reducing drug cinacalcet to elicit different biological responses. The amines formed via intracellular reductive amination were quantified by high performance liquid chromatography, revealing that turnover numbers of up to ∼20 were achieved. This work is expected to enable greater versatility and precision in transforming a wide range of aldehyde-containing entities within living environments, further expanding our biosynthetic chemistry toolbox.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Novel Mannich-Type Multicomponent Reactions: Discovery, Mechanism, and Application.Acc Chem Res. 2025 Jul 15;58(14):2317-2331. doi: 10.1021/acs.accounts.5c00338. Epub 2025 Jul 1. Acc Chem Res. 2025. PMID: 40591930
-
Organic Synthesis Away from Equilibrium: Contrathermodynamic Transformations Enabled by Excited-State Electron Transfer.Acc Chem Res. 2024 Jul 2;57(13):1827-1838. doi: 10.1021/acs.accounts.4c00227. Epub 2024 Jun 21. Acc Chem Res. 2024. PMID: 38905487 Free PMC article.
-
Survivor, family and professional experiences of psychosocial interventions for sexual abuse and violence: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2022 Oct 4;10(10):CD013648. doi: 10.1002/14651858.CD013648.pub2. Cochrane Database Syst Rev. 2022. PMID: 36194890 Free PMC article.
-
Primary Amine-Based Photoclick Chemistry: From Concept to Diverse Applications in Chemical Biology and Medicinal Chemistry.Acc Chem Res. 2025 Jul 1;58(13):1963-1981. doi: 10.1021/acs.accounts.5c00158. Epub 2025 Jun 18. Acc Chem Res. 2025. PMID: 40532071 Review.
References
-
- Wójcik W; Łukasiewicz M; Puppel K Biogenic amines: formation, action and toxicity – a review. J. Sci. Food Agr 2021, 101, 2634–2640. - PubMed
-
- Kourounakis AP; Xanthopoulos D; Tzara A Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev 2020, 40, 709–752. - PubMed
-
- Afanasyev OI; Kuchuk E; Usanov DL; Chusov D Reductive Amination in the Synthesis of Pharmaceuticals. Chem. Rev 2019, 119, 11857–11911. - PubMed
-
- Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

