Gaining insights into epigenetic memories through artificial intelligence and omics science in plants
- PMID: 40552708
- PMCID: PMC12402757
- DOI: 10.1111/jipb.13953
Gaining insights into epigenetic memories through artificial intelligence and omics science in plants
Abstract
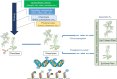
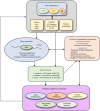
Plants exhibit remarkable abilities to learn, communicate, memorize, and develop stimulus-dependent decision-making circuits. Unlike animals, plant memory is uniquely rooted in cellular, molecular, and biochemical networks, lacking specialized organs for these functions. Consequently, plants can effectively learn and respond to diverse challenges, becoming used to recurring signals. Artificial intelligence (AI) and machine learning (ML) represent the new frontiers of biological sciences, offering the potential to predict crop behavior under environmental stresses associated with climate change. Epigenetic mechanisms, serving as the foundational blueprints of plant memory, are crucial in regulating plant adaptation to environmental stimuli. They achieve this adaptation by modulating chromatin structure and accessibility, which contribute to gene expression regulation and allow plants to adapt dynamically to changing environmental conditions. In this review, we describe novel methods and approaches in AI and ML to elucidate how plant memory occurs in response to environmental stimuli and priming mechanisms. Furthermore, we explore innovative strategies exploiting transgenerational memory for plant breeding to develop crops resilient to multiple stresses. In this context, AI and ML can aid in integrating and analyzing epigenetic data of plant stress responses to optimize the training of the parental plants.
Keywords: DNA methylation; deep learning; gene expression; machine learning; stress memory; transgenerational inheritance.
© 2025 The Author(s). Journal of Integrative Plant Biology published by John Wiley & Sons Australia, Ltd on behalf of Institute of Botany, Chinese Academy of Sciences.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Akdogan, G. , Tufekci, E.D. , Uranbey, S. , and Unver, T. (2016). miRNA‐based drought regulation in wheat. Funct. Integr. Genomics 16: 221–233. - PubMed
-
- Anastasiadi, D. , Venney, C.J. , Bernatchez, L. , and Wellenreuther, M. (2021). Epigenetic inheritance and reproductive mode in plants and animals. Trends. Ecol. Evol. 36: 1124–1140. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

