Clinical effectiveness, safety, and viral mutagenicity of oral favipiravir for COVID-19: results from a community-based, open-label, randomized Phase III trial
- PMID: 40552814
- PMCID: PMC12327002
- DOI: 10.1128/aac.00054-25
Clinical effectiveness, safety, and viral mutagenicity of oral favipiravir for COVID-19: results from a community-based, open-label, randomized Phase III trial
Abstract
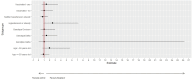
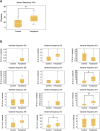
Early community treatment of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection may reduce severe coronavirus disease (COVID-19) incidence. We evaluated clinical effectiveness, safety, and SARS-CoV-2 mutagenicity of favipiravir, an oral viral RNA polymerase inhibitor. We performed an open-label, community-based, randomized Phase III trial, recruiting non-hospitalized adults with mild COVID-19 (WHO ordinal severity score [OSS] ≤ 3). Positive cases were invited to web-based self-screening within 24 h using public health data. Exclusion criteria included symptoms for >7 days, pregnancy/breastfeeding, severe renal/liver disease, gout, and licensed antiviral eligibility. Participants were randomized 1:1 to 10 days favipiravir (Day 1: 3,600 mg; days 2-10: 1,600 mg) or no additional treatment. The primary endpoint was worst recorded OSS up to and including Day 15 (intention-to-treat). The target recruitment was 302. Secondary endpoints included adverse event (AE) rate to Day 60, time-to-viral clearance (TTVC), time-to-symptom resolution (TTSR), and SARS-CoV-2 sequencing variant rate (≥5% frequency) at Day 15 (registration ISRCTN: 31062548; EudraCT: 2020-001904-41). A total of 68,788 adults were invited, and 302 (0.4%) were subsequently randomized between December 2020 and July 2022 (favipiravir [n = 152]: standard care [n = 150]). Mean (SD) age was 47.2 (13.2), and 230/302 (76%) were vaccinated. Severe outcomes were infrequent, with no intensive care unit admissions/deaths. There was no difference in the primary endpoint: odds ratio 1.18 (95% confidence interval [CI] 0.63-2.20), TTSR (HR 1.03 [95% CI 0.81-1.31]), or TTVC (HR 1.13 [95% CI 0.65-1.97]). Favipiravir was well tolerated with few AEs but was associated with increased variant frequency, including C-to-U mutations. Community administration of favipiravir for mild COVID-19 was not associated with clinical benefits or safety concerns but was associated with SARS-CoV-2 mutagenicity.CLINICAL TRIALSThis study is registered with ISRCTN as 31062548 and with EU-CTR as 2020-001904-41.
Keywords: COVID-19; antiviral agents; randomized controlled trial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organisation . 2025. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int
-
- Vo AD, La J, Wu JT-Y, Strymish JM, Ronan M, Brophy M, Do NV, Branch-Elliman W, Fillmore NR, Monach PA. 2022. Factors associated with severe COVID-19 among vaccinated adults treated in US veterans affairs hospitals. JAMA Netw Open 5:e2240037. doi: 10.1001/jamanetworkopen.2022.40037 - DOI - PMC - PubMed
-
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, COVID-19 Genomics UK (COG-UK) Consortium, Peacock SJ, Robertson DL. 2021. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19:409–424. doi: 10.1038/s41579-021-00573-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

