CypA inhibits respiratory syncytial virus (RSV) replication by suppressing glycolysis through the downregulation of PKM2 expression
- PMID: 40552816
- PMCID: PMC12282163
- DOI: 10.1128/jvi.00074-25
CypA inhibits respiratory syncytial virus (RSV) replication by suppressing glycolysis through the downregulation of PKM2 expression
Abstract
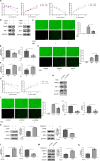
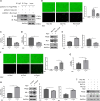
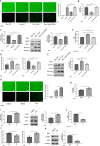
The "Warburg effect," a type of metabolic reprogramming characterized by enhanced glycolysis even in the presence of oxygen, is frequently observed in tumor cells and has also been detected in cells infected with viruses. Our study demonstrated that respiratory syncytial virus (RSV) infection induced aerobic glycolysis both in vivo and in vitro. By utilizing the glycolysis agonist PS48 or inhibitor 2-DG, we ascertained that RSV can utilize glycolysis to promote its replication. Mechanistically, glycolysis may facilitate RSV replication by negatively regulating the IFNβ response. Additionally, we discovered a host molecule, namely CypA, that could downregulate glycolysis to combat RSV infection. CypA interacted with PKM2, a key enzyme of glycolysis, and reduced its expression. By overexpressing or knocking down CypA, we verified that CypA could inhibit aerobic glycolysis, enhance IFNβ production, and reduce RSV replication. Inhibiting the PPIase activity of CypA resulted in the disappearance of its function, indicating that CypA exerted its effects dependent on PPIase activity. Furthermore, we found that CypA has a synergistic effect with 2-DG and an antagonistic effect with PS48 on the IFNβ response, supporting the notion that CypA regulates IFNβ by inhibiting glycolysis. These results indicate that CypA may serve as a novel host factor in the regulation of glycolysis, the interferon response, and ultimately in resisting RSV infection.
Importance: Viruses utilize the host's resources and energy to carry out essential life processes and achieve self-replication. In response, hosts have evolved a range of antagonistic mechanisms. Our study investigates how RSV employs glycolysis to benefit its replication, with a particular focus on the interaction between glycolysis and IFNβ regulation. Additionally, we explore how the host employs CypA to antagonize the virus's utilization of glycolysis, thereby inhibiting RSV replication. Our findings will contribute to the development of effective antiviral therapies targeting CypA.
Keywords: CypA; IFNβ; PKM2; RSV; glycolysis; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

