Evaluation of the efficient propagation of Rhizophagus intraradices and its inoculation effects on rice
- PMID: 40552883
- PMCID: PMC12285234
- DOI: 10.1128/aem.00558-25
Evaluation of the efficient propagation of Rhizophagus intraradices and its inoculation effects on rice
Abstract
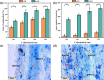
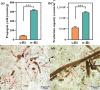
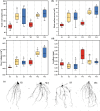
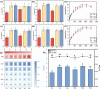
Arbuscular mycorrhizal fungi (AMF) are a key group of fungi closely associated with agricultural production within soil microbial communities. However, large-scale propagation of AMF inoculum faces various challenges, limiting our ability to obtain and utilize these inocula on a broad scale. To address this, we designed a monolayer mesh cultivation system employing a hydroponic approach for propagating arbuscular mycorrhizal fungi, specifically Rhizophagus intraradices. We conducted a comparative analysis of quality and inoculation efficiency between the water culture inoculum (w-Ri) and traditional soil-based inoculum (s-Ri). Our findings revealed the following. (i) The propagation cycle of w-Ri inoculum is 35 days and only 23% of the 150-day cycle required for s-Ri inoculum. (ii) The spore density, viability, and purity of w-Ri inoculum are 5.25 times, 1.09 times, and 1.26 times higher, respectively, than those of s-Ri inoculum. (iii) The w-Ri inoculants demonstrate effects on enhancing rice biomass, root morphology, and photosynthesis that are consistent with those of the s-Ri inoculants, while requiring only 10% of the application rate of the s-Ri inoculants. These results provide crucial theoretical references for establishing a pure and efficient arbuscular mycorrhizal fungus propagation system and its promotion and application.IMPORTANCEThe development of a monolayer mesh hydroponic cultivation system for propagating Rhizophagus intraradices offers a significant advancement in overcoming the challenges of large-scale AMF inoculum production, which is critical for enhancing agricultural sustainability. The comparative analysis of water culture-based (w-Ri) and traditional soil-based (s-Ri) inoculum demonstrates the superior efficiency of the w-Ri system in terms of propagation speed, spore density, and inoculum quality, highlighting its potential for large-scale application in farming practices. The findings that w-Ri inoculants are equally effective in promoting plant growth while requiring only a fraction of the application rate of s-Ri inoculants underscore the potential for reducing both cost and environmental impact in agricultural inoculation practices.
Keywords: arbuscular mycorrhizal fungi; chlorophyll fluorescence parameters; inoculation effects; root morphology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Becker S, Fanzo J. 2023. Population and food systems: what does the future hold? Popul Environ 45:26. doi: 10.1007/s11111-023-00431-6 - DOI
-
- Feldmann F, Schneider C. 2020. Directed inoculum production of arbuscular mycorrhizal fungi - the state of the art. J Appl Bot Food Qual 93:280–288.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

