Acidosis attenuates the hypoxic stabilization of HIF-1α by activating lysosomal degradation
- PMID: 40552983
- PMCID: PMC12187095
- DOI: 10.1083/jcb.202409103
Acidosis attenuates the hypoxic stabilization of HIF-1α by activating lysosomal degradation
Abstract
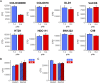
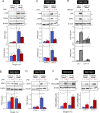
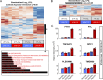
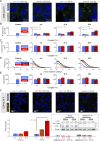
Hypoxia-inducible factors (HIFs) mediate cellular responses to low oxygen, notably enhanced fermentation that acidifies poorly perfused tissues and may eventually become more damaging than adaptive. How pH feeds back on hypoxic signaling is unclear but critical to investigate because acidosis and hypoxia are mechanistically coupled in diffusion-limited settings, such as tumors. Here, we examined the pH sensitivity of hypoxic signaling in colorectal cancer cells that can survive acidosis. HIF-1α stabilization under acidotic hypoxia was transient, declining over 48 h. Proteomic analyses identified responses that followed HIF-1α, including canonical HIF targets (e.g., CA9, PDK1), but these did not reflect a proteome-wide downregulation. Enrichment analyses suggested a role for lysosomal degradation. Indeed, HIF-1α destabilization was blocked by inactivating lysosomes, but not proteasome inhibitors. Acidotic hypoxia stimulated lysosomal activity and autophagy via mammalian target of rapamycin complex I (mTORC1), resulting in HIF-1α degradation. This response protects cells from excessive acidification by unchecked fermentation. Thus, alkaline conditions are permissive for at least some aspects of HIF-1α signaling.
© 2025 White et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

